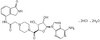
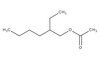
322326 Sigma-AldrichDiphtheria Toxin, Unnicked, Corynebacterium diphtheriae - Calbiochem
Diphtheria toxin catalyzes ADP-ribosylation of eukaryotic aminoacyltransferase II (EF2) using NAD as substrate. Activation requires nicking with a protease followed by reduction with DTT.
More>> Diphtheria toxin catalyzes ADP-ribosylation of eukaryotic aminoacyltransferase II (EF2) using NAD as substrate. Activation requires nicking with a protease followed by reduction with DTT. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 322326-1MGCN |
|
Glass bottle | 1 mg |
|
— |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | Solid |
| Formulation | Lyophilized from sterile 10 mM Tris, 1 mM EDTA, pH 7.5. |
| Reversible | N |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | XW5807200 |
| Safety Information | |
|---|---|
| R Phrase | R: 20/21/22 Harmful by inhalation, in contact with skin and if swallowed. |
| S Phrase | S: 36 Wear suitable protective clothing. |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 322326-1MGCN | 07790788060923 |
Documentation
Diphtheria Toxin, Unnicked, Corynebacterium diphtheriae - Calbiochem SDS
| Title |
|---|
Diphtheria Toxin, Unnicked, Corynebacterium diphtheriae - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 322326 |
References
| Reference overview |
|---|
| Kochi, S.K., and Collier, R.J. 1993. Exp. Cell Res. 208, 296. Chang, M.P., et al. 1989. J. Biol. Chem. 264, 15261. Pappenheimer, A.M., Jr. 1977. Annu. Rev. Biochem. 46, 69. Ittelson, T.R., and Gill, D.M. 1973. Nature 242, 330. Uchida, T., et al. 1973. J. Biol. Chem. 248, 3851. Pappenheimer, A.M., et al. 1972. Immunochem. 9, 891. Uchida, T., et al. 1972. Science 175, 901. Bowman, C.G., and Bonventre, P.F. 1970. J. Exp. Med. 131, 659. Baseman, J.B., et al. 1970. J. Exp. Med. 132, 1138. Gill, D.M., et al. 1969. J. Exp. Med. 129, 1. Gill, D.M., et al. 1969. Cold Spring Harbor Symp. Quant. Biol. 34, 589. Honjo, J., et al. 1968. J. Biol. Chem. 243, 3553. |