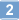Preclinical Testing Oncolytic Vaccinia Virus Strain GLV-5b451 Expressing an Anti-VEGF Single-Chain Antibody for Canine Cancer Therapy.
Adelfinger, M; Bessler, S; Frentzen, A; Cecil, A; Langbein-Laugwitz, J; Gentschev, I; Szalay, AA
Viruses
7
4075-92
2015
Show Abstract
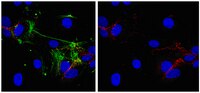
Virotherapy on the basis of oncolytic vaccinia virus (VACV) strains is a novel approach for canine cancer therapy. Here we describe, for the first time, the characterization and the use of VACV strain GLV-5b451 expressing the anti-vascular endothelial growth factor (VEGF) single-chain antibody (scAb) GLAF-2 as therapeutic agent against different canine cancers. Cell culture data demonstrated that GLV-5b451 efficiently infected and destroyed all four tested canine cancer cell lines including: mammary carcinoma (MTH52c), mammary adenoma (ZMTH3), prostate carcinoma (CT1258), and soft tissue sarcoma (STSA-1). The GLV-5b451 virus-mediated production of GLAF-2 antibody was observed in all four cancer cell lines. In addition, this antibody specifically recognized canine VEGF. Finally, in canine soft tissue sarcoma (CSTS) xenografted mice, a single systemic administration of GLV-5b451 was found to be safe and led to anti-tumor effects resulting in the significant reduction and substantial long-term inhibition of tumor growth. A CD31-based immuno-staining showed significantly decreased neo-angiogenesis in GLV-5b451-treated tumors compared to the controls. In summary, these findings indicate that GLV-5b451 has potential for use as a therapeutic agent in the treatment of CSTS. | | | 26205404
 |
Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects.
Cardoso, FL; Herz, J; Fernandes, A; Rocha, J; Sepodes, B; Brito, MA; McGavern, DB; Brites, D
Journal of neuroinflammation
12
82
2015
Show Abstract
The inflammatory mediator lipopolysaccharide (LPS) has been shown to induce acute gliosis in neonatal mice. However, the progressive effects on the murine neurodevelopmental program over the week that follows systemic inflammation are not known. Thus, we investigated the effects of repeated LPS administration in the first postnatal week in mice, a condition mimicking sepsis in late preterm infants, on the developing central nervous system (CNS).Systemic inflammation was induced by daily intraperitoneal administration (i.p.) of LPS (6 mg/kg) in newborn mice from postnatal day (PND) 4 to PND6. The effects on neurodevelopment were examined by staining the white matter and neurons with Luxol Fast Blue and Cresyl Violet, respectively. The inflammatory response was assessed by quantifying the expression/activity of matrix metalloproteinases (MMP), toll-like receptor (TLR)-4, high mobility group box (HMGB)-1, and autotaxin (ATX). In addition, B6 CX3CR1(gfp/+) mice combined with cryo-immunofluorescence were used to determine the acute, delayed, and lasting effects on myelination, microglia, and astrocytes.LPS administration led to acute body and brain weight loss as well as overt structural changes in the brain such as cerebellar hypoplasia, neuronal loss/shrinkage, and delayed myelination. The impaired myelination was associated with alterations in the proliferation and differentiation of NG2 progenitor cells early after LPS administration, rather than with excessive phagocytosis by CNS myeloid cells. In addition to disruptions in brain architecture, a robust inflammatory response to LPS was observed. Quantification of inflammatory biomarkers revealed decreased expression of ATX with concurrent increases in HMGB1, TLR-4, and MMP-9 expression levels. Acute astrogliosis (GFAP(+) cells) in the brain parenchyma and at the microvasculature interface together with parenchymal microgliosis (CX3CR1(+) cells) were also observed. These changes preceded the migration/proliferation of CX3CR1(+) cells around the vessels at later time points and the subsequent loss of GFAP(+) astrocytes.Collectively, our study has uncovered a complex innate inflammatory reaction and associated structural changes in the brains of neonatal mice challenged peripherally with LPS. These findings may explain some of the neurobehavioral abnormalities that develop following neonatal sepsis. | | | 25924675
 |
Degeneration and regeneration of corneal nerves in response to HSV-1 infection.
Chucair-Elliott, AJ; Zheng, M; Carr, DJ
Investigative ophthalmology & visual science
56
1097-107
2015
Show Abstract
Herpes simplex virus type 1 (HSV-1) infection is one cause of neurotrophic keratitis, characterized by decreases in corneal sensation, blink reflex, and tear secretion as consequence of damage to the sensory fibers innervating the cornea. Our aim was to characterize changes in the corneal nerve network and its function in response to HSV-1 infection.C57BL/6J mice were infected with HSV-1 or left uninfected. Corneas were harvested at predetermined times post infection (pi) and assessed for β III tubulin, substance P, calcitonin gene-related peptide, and neurofilament H staining by immunohistochemistry (IHC). Corneal sensitivity was evaluated using a Cochet-Bonnet esthesiometer. Expression of genes associated with nerve repair was determined in corneas by real time RT-PCR, Western blotting, and IHC. Semaphorin 7A (SEMA 7A) neutralizing antibody or isotype control was subconjunctivally administered to infected mice.The area of cornea occupied by β III tubulin immunoreactivity and sensitivity significantly decreased by day 8 pi. Modified reinnervation was observed by day 30 pi without recovery of corneal sensation. Sensory fibers were lost by day 8 pi and were still absent or abnormal at day 30 pi. Expression of SEMA 7A increased at day 8 pi, localizing to corneal epithelial cells. Neutralization of SEMA 7A resulted in defective reinnervation and lower corneal sensitivity.Corneal sensory nerves were lost, consistent with loss of corneal sensation at day 8 pi. At day 30 pi, the cornea reinnervated but without recovering the normal arrangement of its fibers or function. SEMA 7A expression was increased at day 8pi, likely as part of a nerve regeneration mechanism. | | | 25587055
 |
Endothelial destabilization by angiopoietin-2 via integrin β1 activation.
Hakanpaa, L; Sipila, T; Leppanen, VM; Gautam, P; Nurmi, H; Jacquemet, G; Eklund, L; Ivaska, J; Alitalo, K; Saharinen, P
Nature communications
6
5962
2015
Show Abstract
Angiopoietins regulate vascular homeostasis via the endothelial Tie receptor tyrosine kinases. Angiopoietin-1 (Ang1) supports endothelial stabilization via Tie2 activation. Angiopoietin-2 (Ang2) functions as a context-dependent Tie2 agonist/antagonist promoting pathological angiogenesis, vascular permeability and inflammation. Elucidating Ang2-dependent mechanisms of vascular destablization is critical for rational design of angiopoietin antagonists that have demonstrated therapeutic efficacy in cancer trials. Here, we report that Ang2, but not Ang1, activates β1-integrin, leading to endothelial destablization. Autocrine Ang2 signalling upon Tie2 silencing, or in Ang2 transgenic mice, promotes β1-integrin-positive elongated matrix adhesions and actin stress fibres, regulating vascular endothelial-cadherin-containing cell-cell junctions. The Tie2-silenced monolayer integrity is rescued by β1-integrin, phosphoinositide-3 kinase or Rho kinase inhibition, and by re-expression of a membrane-bound Tie2 ectodomain. Furthermore, Tie2 silencing increases, whereas Ang2 blocking inhibits transendothelial tumour cell migration in vitro. These results establish Ang2-mediated β1-integrin activation as a promoter of endothelial destablization, explaining the controversial vascular functions of Ang1 and Ang2. | Immunofluorescence | | 25635707
 |
Local acting Sticky-trap inhibits vascular endothelial growth factor dependent pathological angiogenesis in the eye.
Michael, IP; Westenskow, PD; Hacibekiroglu, S; Greenwald, AC; Ballios, BG; Kurihara, T; Li, Z; Warren, CM; Zhang, P; Aguilar, E; Donaldson, L; Marchetti, V; Baba, T; Hussein, SM; Sung, HK; Iruela-Arispe, ML; Rini, JM; van der Kooy, D; Friedlander, M; Nagy, A
EMBO molecular medicine
6
604-23
2014
Show Abstract
Current therapeutic antiangiogenic biologics used for the treatment of pathological ocular angiogenesis could have serious side effects due to their interference with normal blood vessel physiology. Here, we report the generation of novel antivascular endothelial growth factor-A (VEGF) biologics, termed VEGF "Sticky-traps," with unique properties that allow for local inhibition of angiogenesis without detectable systemic side effects. Using genetic and pharmacological approaches, we demonstrated that Sticky-traps could locally inhibit angiogenesis to at least the same extent as the original VEGF-trap that also gains whole-body access. Sticky-traps did not cause systemic effects, as shown by uncompromised wound healing and normal tracheal vessel density. Moreover, if injected intravitreally, recombinant Sticky-trap remained localized to various regions of the eye, such as the inner-limiting membrane and ciliary body, for prolonged time periods, without gaining access either to the photoreceptors/choriocapillaris area or the circulation. These unique pharmacological characteristics of Sticky-trap could allow for safe treatment of pathological angiogenesis in patients with diabetic retinopathy and retinopathy of pre-maturity. | | | 24705878
 |
The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel.
Aspelund, A; Tammela, T; Antila, S; Nurmi, H; Leppänen, VM; Zarkada, G; Stanczuk, L; Francois, M; Mäkinen, T; Saharinen, P; Immonen, I; Alitalo, K
The Journal of clinical investigation
124
3975-86
2014
Show Abstract
In glaucoma, aqueous outflow into the Schlemm's canal (SC) is obstructed. Despite striking structural and functional similarities with the lymphatic vascular system, it is unknown whether the SC is a blood or lymphatic vessel. Here, we demonstrated the expression of lymphatic endothelial cell markers by the SC in murine and zebrafish models as well as in human eye tissue. The initial stages of SC development involved induction of the transcription factor PROX1 and the lymphangiogenic receptor tyrosine kinase VEGFR-3 in venous endothelial cells in postnatal mice. Using gene deletion and function-blocking antibodies in mice, we determined that the lymphangiogenic growth factor VEGF-C and its receptor, VEGFR-3, are essential for SC development. Delivery of VEGF-C into the adult eye resulted in sprouting, proliferation, and growth of SC endothelial cells, whereas VEGF-A obliterated the aqueous outflow system. Furthermore, a single injection of recombinant VEGF-C induced SC growth and was associated with trend toward a sustained decrease in intraocular pressure in adult mice. These results reveal the evolutionary conservation of the lymphatic-like phenotype of the SC, implicate VEGF-C and VEGFR-3 as critical regulators of SC lymphangiogenesis, and provide a basis for further studies on therapeutic manipulation of the SC with VEGF-C in glaucoma treatment. | Immunofluorescence | Mouse | 25061878
 |
Formulation optimization and in vivo proof-of-concept study of thermosensitive liposomes balanced by phospholipid, elastin-like polypeptide, and cholesterol.
Park, SM; Cha, JM; Nam, J; Kim, MS; Park, SJ; Park, ES; Lee, H; Kim, HR
PloS one
9
e103116
2014
Show Abstract
One application of nanotechnology in medicine that is presently being developed involves a drug delivery system (DDS) employing nanoparticles to deliver drugs to diseased sites in the body avoiding damage of healthy tissue. Recently, the mild hyperthermia-triggered drug delivery combined with anticancer agent-loaded thermosensitive liposomes was widely investigated. In this study, thermosensitive liposomes (TSLs), composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-PEG), cholesterol, and a fatty acid conjugated elastin-like polypeptide (ELP), were developed and optimized for triggered drug release, controlled by external heat stimuli. We introduced modified ELP, tunable for various biomedical purposes, to our thermosensitive liposome (e-TSL) to convey a high thermoresponsive property. We modulated thermosensitivity and stability by varying the ratios of e-TSL components, such as phospholipid, ELP, and cholesterol. Experimental data obtained in this study corresponded to results from a simulation study that demonstrated, through the calculation of the lateral diffusion coefficient, increased permeation of the lipid bilayer with higher ELP concentrations, and decreased permeation in the presence of cholesterol. Finally, we identified effective drug accumulation in tumor tissues and antitumor efficacy with our optimized e-TSL, while adjusting lag-times for systemic accumulation. | | | 25068721
 |
Nerve growth factor regulates neurolymphatic remodeling during corneal inflammation and resolution.
Fink, DM; Connor, AL; Kelley, PM; Steele, MM; Hollingsworth, MA; Tempero, RM
PloS one
9
e112737
2014
Show Abstract
The cellular and physiologic mechanisms that regulate the resolution of inflammation remain poorly defined despite their widespread importance in improving inflammatory disease outcomes. We studied the resolution of two cardinal signs of inflammation-pain and swelling-by investigating molecular mechanisms that regulate neural and lymphatic vessel remodeling during the resolution of corneal inflammation. A mouse model of corneal inflammation and wound recovery was developed to study this process in vivo. Administration of nerve growth factor (NGF) increased pain sensation and inhibited neural remodeling and lymphatic vessel regression processes during wound recovery. A complementary in vivo approach, the corneal micropocket assay, revealed that NGF-laden pellets stimulated lymphangiogenesis and increased protein levels of VEGF-C. Adult human dermal lymphatic endothelial cells did not express canonical NGF receptors TrkA and p75NTR or activate downstream MAPK- or Akt-pathway effectors in the presence of NGF, although NGF treatment increased their migratory and tubulogenesis capacities in vitro. Blockade of the VEGF-R2/R3 signaling pathway ablated NGF-mediated lymphangiogenesis in vivo. These findings suggest a hierarchical relationship with NGF functioning upstream of the VEGF family members, particularly VEGF-C, to stimulate lymphangiogenesis. Taken together, these studies show that NGF stimulates lymphangiogenesis and that NGF may act as a pathogenic factor that negatively regulates the normal neural and lymphatic vascular remodeling events that accompany wound recovery. | | | 25383879
 |
Ocular phenotype of Fbn2-null mice.
Shi, Y; Tu, Y; Mecham, RP; Bassnett, S
Investigative ophthalmology & visual science
54
7163-73
2013
Show Abstract
Fibrillin-2 (Fbn2) is the dominant fibrillin isoform expressed during development of the mouse eye. To test its role in morphogenesis, we examined the ocular phenotype of Fbn2(-/-) mice.Ocular morphology was assessed by confocal microscopy using antibodies against microfibril components.Fbn2(-/-) mice had a high incidence of anterior segment dysgenesis. The iris was the most commonly affected tissue. Complete iridal coloboma was present in 37% of eyes. Dyscoria, corectopia and pseudopolycoria were also common (43% combined incidence). In wild-type (WT) mice, fibrillin-2-rich microfibrils are prominent in the pupillary membrane (PM) during development. In Fbn2-null mice, the absence of Fbn2 was partially compensated for by increased expression of fibrillin-1, although the resulting PM microfibrils were disorganized, compared with WTs. In colobomatous adult Fbn2(-/-) eyes, the PM failed to regress normally, especially beneath the notched region of the iris. Segments of the ciliary body were hypoplastic, and zonular fibers, although relatively plentiful, were unevenly distributed around the lens equator. In regions where the zonular fibers were particularly disturbed, the synchronous differentiation of the underlying lens fiber cells was affected.Fbn2 has an indispensable role in ocular morphogenesis in mice. The high incidence of iris coloboma in Fbn2-null animals implies a previously unsuspected role in optic fissure closure. The observation that fiber cell differentiation was disturbed in Fbn2(-/-) mice raises the possibility that the attachment of zonular fibers to the lens surface may help specify the equatorial margin of the lens epithelium. | | | 24130178
 |
Mitochondrial oxidative phosphorylation reserve is required for hormone- and PPARγ agonist-induced adipogenesis.
Ryu, MJ; Kim, SJ; Choi, MJ; Kim, YK; Lee, MH; Lee, SE; Chung, HK; Jung, SB; Kim, HJ; Kim, KS; Jo, YS; Kweon, GR; Lee, CH; Shong, M
Molecules and cells
35
134-41
2013
Show Abstract
Adipocyte differentiation requires the coordinated activities of several nuclear transcription factors. Recently, mitochondria biogenesis was reported to occur during adipocyte differentiation and following treatment with thiazolidinediones in vitro and in vivo. Crif1 is a translational factor for mitochondrial DNA (mtDNA) and is important for transcription of the mitochondrial oxidative phosphorylation (OXPHOS) complex. To investigate the role of OXPHOS in adipogenesis, we analyzed adipocyte differentiation following disruption of Crif1 in vitro and in vivo. The adipose-specific Crif1 knockout mouse had a lower body weight and less fat mass than wild-type mice. Furthermore, adipocytes were smaller and had a dysplastic morphology in the adipose-specific Crif1 knockout mouse. 3T3-L1 adipocytes or adipose-derived stem cells (ADSCs) that lacked Crif1 expressed lower levels of mtDNA-encoded OXPHOS subunits, and adipocyte differentiation was disrupted. Rosiglitazone treatment did not induce adipogenesis or mitochondria biogenesis in Crif1 knockout ADSCs. These results show that mitochondrial OXPHOS and Crif1 are required for rosiglitazone- and hormone-induced adipogenesis. | | | 23456335
 |