324518 Sigma-AldricheIF4E/eIF4G Interaction Inhibitor II, 4E1RCat - Calbiochem
The eIF4E/eIF4G Interaction Inhibitor II, 4E1RCat controls the biological activity of eIF4E/eIF4G interaction. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The eIF4E/eIF4G Interaction Inhibitor II, 4E1RCat controls the biological activity of eIF4E/eIF4G interaction. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<同義語: eIF4F Inhibitor II, 4-((3E)-3-((5-(4-Nitrophenyl)furan-2-yl)methylidene)-2-oxo-5-phenyl-2,3-dihydro-1H-pyrrol-1-yl)benzoic acid
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| Empirical Formula |
|---|
| C₂₈H₁₈N₂O₆ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 324518-10MGCN |
|
ガラスビン | 10 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable oxopyrrolyl benzoate compound that blocks the assembly of eukaryotic translation initiation factor (eIF) 4F complex (eIF4F) as well as disrupts preformed eIF4F by competing against eIF4G for eIF4E binding. Specifically blocks cap-dependent, but not cap-independent, protein translations in cell-free translation assays and inhibits protein, but not DNA or RNA, synthesis in MDA-MB-231 and HeLa cultures (50 µM) in a reversible manner. Although not effective when administered alone (15 mg/kg, i.p. daily for 5 days), 4E1RCat is shown to significantly prolong Pten+/-Eµ-myc and Tsc+/-Eµ-myc, but not Eµ-myc, tumor remission time when combined with Doxorubicin (Cat. No. 324380) treatment (10 mg/kg, i.p. once on day 2) in mice in vivo. Unlike 4EGI-1 (Cat. No. 324517), 4E1RCat also blocks eIF4E and 4E-BP1 interaction. |
| Catalogue Number | 324518 |
| Brand Family | Calbiochem® |
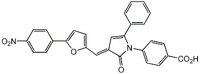
| Synonyms | eIF4F Inhibitor II, 4-((3E)-3-((5-(4-Nitrophenyl)furan-2-yl)methylidene)-2-oxo-5-phenyl-2,3-dihydro-1H-pyrrol-1-yl)benzoic acid |
| References | |
|---|---|
| References | Cencic, R., et al. 2011. Proc. Natl. Acad. Sci. USA 108, 1046. |
| Product Information | |
|---|---|
| Form | Dark brown solid |
| Hill Formula | C₂₈H₁₈N₂O₆ |
| Chemical formula | C₂₈H₁₈N₂O₆ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 324518-10MGCN | 04055977197334 |
Documentation
eIF4E/eIF4G Interaction Inhibitor II, 4E1RCat - Calbiochem (M)SDS
| タイトル |
|---|
eIF4E/eIF4G Interaction Inhibitor II, 4E1RCat - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 324518 |
参考資料
| 参考資料の概要 |
|---|
| Cencic, R., et al. 2011. Proc. Natl. Acad. Sci. USA 108, 1046. |