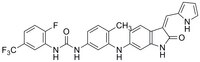
648451 Sigma-AldrichTrk Inhibitor III, GNF-5837 - CAS 1033769-28-6 - Calbiochem
The Trk Inhibitor III, GNF-5837 controls the biological activity of Trk. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The Trk Inhibitor III, GNF-5837 controls the biological activity of Trk. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<同義語: (Z)-1-(3-((3-((1H-Pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)amino)-4-methylphenyl)-3-(2-fluoro-5-(trifluoromethyl)phenyl)urea
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
| 1033769-28-6 | C₂₈H₂₁F₄N₅O₂ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 648451-10MGCN |
|
ガラスビン | 10 mg |
|
— |
| References | |
|---|---|
| References | Albaugh, P., et al. 2012. ACS Med. Chem. Lett. 3, In press. |
| Product Information | |
|---|---|
| CAS number | 1033769-28-6 |
| Form | Orange powder |
| Hill Formula | C₂₈H₂₁F₄N₅O₂ |
| Chemical formula | C₂₈H₂₁F₄N₅O₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 648451-10MGCN | 04055977262377 |
Documentation
Trk Inhibitor III, GNF-5837 - CAS 1033769-28-6 - Calbiochem (M)SDS
| タイトル |
|---|
Trk Inhibitor III, GNF-5837 - CAS 1033769-28-6 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 648451 |
参考資料
| 参考資料の概要 |
|---|
| Albaugh, P., et al. 2012. ACS Med. Chem. Lett. 3, In press. |