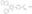616461 Sigma-AldrichTGF-β RI Kinase Inhibitor VI, SB431542 - CAS 301836-41-9 - Calbiochem
TGF-β RI Kinase Inhibitor VI, SB431542, CAS 301836-41-9, is a cell-permeable inhibitor of SMAD2 phosphorylation. Inhibits the activity of ALK4 and ALK5 (IC₅₀ = 140 nM and 94 nM, respectively).
More>> TGF-β RI Kinase Inhibitor VI, SB431542, CAS 301836-41-9, is a cell-permeable inhibitor of SMAD2 phosphorylation. Inhibits the activity of ALK4 and ALK5 (IC₅₀ = 140 nM and 94 nM, respectively). Less<<同義語: SB-431542, 4-[4-(3,4-Methylenedioxyphenyl)-5-(2-pyridyl)-1H-imidazol-2-yl]benzamide, Dihydrate, 4-[4-(1,3-Benzodioxol-5-yl)-5-(2-pyridyl)-1H-imidazol-2-yl]benzamide, Dihydrate
お勧めの製品
-

04-902 Sigma-Aldrich Anti-HA Tag Antibody, clone DW2, rabbit monoclonal -

ZDCD0F05Q ZDCD0F05Q -

475899 Millipore MOPS, Sodium, ULTROL® Grade - CAS 71119-22-7 - Calbiochem -

NA12/31-212 Millipore NovAseptic Valve, Valve Body, Shut off 90˚ TC -
420322 Millipore IPTG, Dioxane-Free, High Purity - CAS 367-93-1 - Calbiochem -

MABC1120-100UG Sigma-Aldrich Anti-PD-L2 Antibody, clone 366C.9E5
概要
主要スペック表
| CAS # | Empirical Formula |
|---|---|
| 301836-41-9 | C₂₂H₁₆N₄O₃. 2H₂O |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 616461-5MGCN |
|
5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable triarylimidazole compound that is shown to effectively inhibit cellular Smad2 phosphorylation (>90% inhibition by 10 µM inhibitor) upon vector-mediated expression of constitutively active ALK4, ALK5, or ALK7 in NIH 3T3 cells, while exhibiting little effect against Smad1 phosphorylation by other members of type I receptors for TGF-β in NIH 3T3 cultures expressing active ALK1, 2, 3, or 6. When tested directly in cell-free kinase assays, SB431542 is demonstrated to potently inhibit the activity of ALK4 and ALK5 (IC50 = 140 nM and 94 nM, respectively) with no or much reduced potency toward a panel of 24 other kinases (IC50 ≥10 µM in the presence of 10 µM ATP), including ALK2 and ALK6. Reported to improve the efficiency of 4-TF-induced human iPSCs generation from fibroblast cultures by >200-fold when used together with PD0325901 (Cat. No. 444966) and Thiazovivin (Cat. No. 420220). Also available as a 100 mM solution in DMSO (Cat. No. 616464). |
| Catalogue Number | 616461 |
| Brand Family | Calbiochem® |
| Synonyms | SB-431542, 4-[4-(3,4-Methylenedioxyphenyl)-5-(2-pyridyl)-1H-imidazol-2-yl]benzamide, Dihydrate, 4-[4-(1,3-Benzodioxol-5-yl)-5-(2-pyridyl)-1H-imidazol-2-yl]benzamide, Dihydrate |
| Product Information | |
|---|---|
| CAS number | 301836-41-9 |
| Form | Off-white solid |
| Hill Formula | C₂₂H₁₆N₄O₃. 2H₂O |
| Chemical formula | C₂₂H₁₆N₄O₃. 2H₂O |
| Structure formula Image | |
| Quality Level | MQ300 |
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC |
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 616461-5MGCN | 04055977185928 |