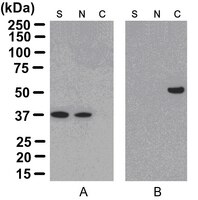
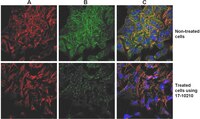
17-10195 Sigma-AldrichProteoExtract® Cytoskeleton Enrichment and Isolation Kit
This kit provides cytoskeleton purification detergent buffers, sufficient for extraction from ten 100 mm culture dishes, that retain focal adhesion & actin-associated proteins while removing soluble cytoplasmic & nuclear proteins from the cell.
More>> This kit provides cytoskeleton purification detergent buffers, sufficient for extraction from ten 100 mm culture dishes, that retain focal adhesion & actin-associated proteins while removing soluble cytoplasmic & nuclear proteins from the cell. Less<<お勧めの製品
概要
| Replacement Information |
|---|
| Description | |
|---|---|
| Catalogue Number | 17-10195 |
| Trade Name |
|
| Description | ProteoExtract® Cytoskeleton Enrichment and Isolation Kit |
| Overview | Read our application note in Nature Methods! http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8624.pdf (Click Here!) Rediscovering the Actin Cytoskeleton: New techniques leading to discoveries in cell migration, Dr. Richard Klemke, Morris Cancer Center, UCSD, USA. The actin cytoskeleton is a highly dynamic network composed of actin polymers and a large variety of associated proteins. The functions of the actin cytoskeleton is to mediate a variety of essential biological functions in all eukaryotic cells, including intra- and extra-cellular movement and structural Support (Chen, C.S., et al., 2003; Frixione E., 2000). To perform these functions, the organization of the actin cytoskeleton must be tightly regulated both temporally and spatially. Many proteins associated with the actin cytoskeleton are thus likely targets of signaling pathways controlling actin assembly. Actin cytoskeleton assembly is regulated at multiple levels, including the organization of actin monomers (G-actin) into actin polymers and the superorganization of actin polymers into a filamentous network (F-actin – the major constituent of microfilaments) (Bretscher, A., et al., 1994). This superorganization of actin polymers into a filamentous network is mediated by actin side-binding or cross-linking proteins (Dubreuil, R. R., 1991; Matsudaira, P., 1991; Matsudaira, P., 1994). The actin cytoskeleton is a dynamic structure that rapidly changes shape and organization in response to stimuli and cell cycle progression. Therefore, a disruption of its normal regulation may lead to cell transformations and cancer. Transformed cells have been shown to contain less F-actin than untransformed cells and exhibit atypical coordination of F-actin levels throughout the cell cycle (Rao, J.Y., et al., 1990). Orientational distribution of actin filaments within a cell is, therefore, an important determinant of cellular shape and motility. Focal adhesion and adherens junctions are membrane-associated complexes that serve as nucleation sites for actin filaments and as cross-linkers between the cell exterior, plasma membrane and actin cytoskeleton (Yamada, K.M., Geiger, B., 1997). The function of focal adhesions is structural, linking the ECM on the outside to the actin cytoskeleton on the inside. They are also sites of signal transduction, initiating signaling pathways in response to adhesion. Focal adhesions consist of integrin-type receptors that are attached to the extracellular matrix and are intracellularly associated with protein complexes containing vinculin (universal focal adhesion marker), talin, α-actinin, paxillin, tensin, zyxin and focal adhesion kinase (FAK) (Burridge, K., et al., 1990; Turner, C.E., Burridge, K., 1991). Studying the proteins that associate with and regulate the actin cytoskeleton has been traditionally difficult because of the insolubility of the cytoskeleton in traditional detergents like Triton-X100. Work over the years has shown that many actin regulatory proteins/phospho-proteins upon activation move from the soluble cytoplasmic compartment to the insoluble actin cytoskeleton. The insolubility of these important proteins has made it difficult to study their biochemical changes, such as phosphorylation and nitrosylation, upon binding to actin. What is sorely needed is a cytoskeleton purification method that allows for the selective enrichment of cytoskeleton-associated proteins for detailed protein biochemical analyses. Such a method would provide the means to directly study this important pool of proteins in normal and diseased cytoskeletons. |
| Materials Required but Not Delivered | 1. Pipettors and tips capable of accurately measuring 1-1000 μL 2. Graduated serological pipettes 3. Microcentrifuge tubes 4. Mechanical vortex 5. Distilled or deionized water 6. 1x Dulbecco’s Phosphate Buffered Saline (D-PBS) 7. 3% Non-fat dry milk diluted in 1x TBST (containing 0.05% Tween-20). |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Quality Level | MQ100 |
| Biological Information |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 15 reactions (1 kit) |
| Material Package | Enough Reagents for 15 Reactions/Isolations |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 17-10195 | 04053252001345 |
Documentation
ProteoExtract® Cytoskeleton Enrichment and Isolation Kit (M)SDS
| タイトル |
|---|
カタログ
| タイトル |
|---|
| 安定したタンパク質抽出と効率的なサンプル調整に Buster & Protein Extraction Kit シリーズとタンパク質調整関連製品 |
ユーザーガイド
| タイトル |
|---|
| ProteoExtract® Cytoskeleton Enrichment and Isolation Kit |
Newsletters / Publications
| Title |
|---|
| Cellutions - The newsletter for Cell Biology Researchers Volume 3: 2011 |