239807 Sigma-AldrichInSolution™ Cyclopamine-KAAD - Calbiochem
InSolution™ Cyclopamine-KAAD, CAS 306387-90-6, is a 1 mM solution in DMSO. A cell-permeable, potent analog of Cyclopamine. Inhibits Hedgehog signaling (IC50 = 20 nM) with lower toxicity.
More>> InSolution™ Cyclopamine-KAAD, CAS 306387-90-6, is a 1 mM solution in DMSO. A cell-permeable, potent analog of Cyclopamine. Inhibits Hedgehog signaling (IC50 = 20 nM) with lower toxicity. Less<<同義語: 3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| Empirical Formula |
|---|
| C₄₄H₆₃Nl₃O₄ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 239807-50UGCN |
|
ガラスビン | 50 μg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable potent analog of Cyclopamine (Cat. No. 239803) that specifically inhibits Hedgehog (Hh) signaling with similar or lower toxicity (IC50 = 20 nM in Shh-LIGHT2 assay; 50 nM in p2Ptch-/-cells; 500 nM in SmoA1-LIGHT cells). Binds to SmoA1 and promotes its exit from the endoplasmic reticulum. Suppresses both ShhNp-induced pathway activity and SmoA1-induced reporter activity. Reported to cause regression of murine tumor allografts in vivo and induce rapid cell death in human medulloblastoma. Shown to sensitize human glioma cells to TRAIL-induced apoptosis. The solid form of this compound (Cat. No. 239804) is also available. |
| Catalogue Number | 239807 |
| Brand Family | Calbiochem® |
| Synonyms | 3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | A 1 mM (50 µg/72 µL) solution of Cyclopamine-KAAD (Cat. No. 239804) in DMSO. |
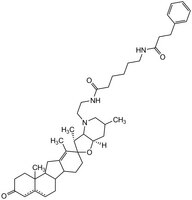
| Hill Formula | C₄₄H₆₃Nl₃O₄ |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Primary Target | SmoA1 |
| Purity | ≥70% by HPLC (sum of two isomers) |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 239807-50UGCN | 04055977198928 |
Documentation
InSolution™ Cyclopamine-KAAD - Calbiochem (M)SDS
| タイトル |
|---|
InSolution™ Cyclopamine-KAAD - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 239807 |
参考資料
| 参考資料の概要 |
|---|
| Siegelin, M.D. et al. 2009. Neurobiol. Dis. 34, 259. Watkins, D.N., et al. 2003. Nature 422, 313. Berman, D.M., et al. 2002. Science 297, 1559. Chen, J.K., et al. 2002. Proc. Natl. Acad. Sci. USA 99, 14071. Chen, J.K., et al. 2002. Genes Dev. 16, 2743. Frank-Kamenetsky, M., et al. 2002. J. Biol. 1, 10. Taipale, J., et al. 2000. Nature 406, 1005. |
| データシート | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|