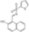
412510 Sigma-AldrichIRE1 Inhibitor I, STF-083010 - Calbiochem
IRE1 Inhibitor I, STF-083010, CAS 307543-71-1, is a cell-permeable compound that directly targets Ireα1 and disrupt Ire1-XBP1 unfolded protein response pathway in RPMI8226 multiple myeloma cells.
More>> IRE1 Inhibitor I, STF-083010, CAS 307543-71-1, is a cell-permeable compound that directly targets Ireα1 and disrupt Ire1-XBP1 unfolded protein response pathway in RPMI8226 multiple myeloma cells. Less<<同義語: N-[(2-Hydroxynaphthalen-1-yl)methylidene]thiophene-2-sulfonamide, N-[(2-Hydroxy-1-naphthyl)methylene]-2-thiophenesulfonamide, ER-to-Nucleus Signaling 1 Inhibitor I, ERN1 Inhibitor I, Inositol-Reguiring Protein 1 Inhibitor I, STF083010
お勧めの製品
-

C5940 ミリスタックプラス(Millistak+®)CE スタックド ディスクフィルター -
807484 Sigma-Aldrich ポリエチレン グリコール 300 -

324681 Sigma-Aldrich Elastase, Human Neutrophil -

110275 Millipore VRBD (VRBG)寒天培地 -

GM10/15-326 Millipore MH GMP1000 WITH EB, EP, DC -

ABN2260-25UG Sigma-Aldrich Anti-alpha-synuclein N103 fragment -

SCWP14250 Millipore MF-ミリポア(MF-Millipore)メンブレン, セルロース混合エステル, 親水性, 8.0 µm, 142 mm, 白色, 無地
概要
主要スペック表
| Empirical Formula |
|---|
| C₁₅H₁₁NO₃S₂ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 412510-10MGCN |
|
ガラスビン | 10 mg |
|
— |
| References | |
|---|---|
| References | Lerner, A.G., et al. 2012 Cell Metabolism 16, 250. Papandreou, I., et al. 2010. Blood 117, )1311. |
| Product Information | |
|---|---|
| Form | Yellow powder |
| Hill Formula | C₁₅H₁₁NO₃S₂ |
| Chemical formula | C₁₅H₁₁NO₃S₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 412510-10MGCN | 04055977188165 |