382149 Sigma-AldrichHistone Deacetylase Inhibitor III
The Histone Deacetylase Inhibitor III, also referenced under CAS 251456-60-7, controls the biological activity of Histone Deacetylase. This small molecule/inhibitor is primarily used for Cell Structure applications.
More>> The Histone Deacetylase Inhibitor III, also referenced under CAS 251456-60-7, controls the biological activity of Histone Deacetylase. This small molecule/inhibitor is primarily used for Cell Structure applications. Less<<同義語: M344, 4-Dimethylamino-N-(6-hydroxycarbamoylhexyl)benzamide, N-Hydroxy-7-(4-dimethylaminobenzoyl)aminoheptanamide
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
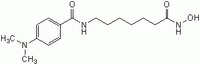
| 251456-60-7 | C₁₆H₂₅N₃O₃ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 382149-1MGCN |
|
樹脂アンプル | 1 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable amide analog of Trichostatin A (Cat. No. 647925) that potently inhibits histone deacetylases (IC50 = 40 nM for rat liver HDAC and IC50 = 100 nM for maize HD). Induces differentiation and inhibits proliferation (~2 µM) of murine erythroleukemia cells. |
| Catalogue Number | 382149 |
| Brand Family | Calbiochem® |
| Synonyms | M344, 4-Dimethylamino-N-(6-hydroxycarbamoylhexyl)benzamide, N-Hydroxy-7-(4-dimethylaminobenzoyl)aminoheptanamide |
| References | |
|---|---|
| References | Ramiszewski, S.W., et al. 2002. J. Med. Chem. 45, 753. Jung, M., et al. 1999. J. Med. Chem. 42, 4669. |
| Product Information | |
|---|---|
| CAS number | 251456-60-7 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₆H₂₅N₃O₃ |
| Chemical formula | C₁₆H₂₅N₃O₃ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Rat liver HDAC |
| Primary Target IC<sub>50</sub> | 40 nM for rat liver HDAC |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 382149-1MGCN | 04055977213164 |
Documentation
Histone Deacetylase Inhibitor III (M)SDS
| タイトル |
|---|
Histone Deacetylase Inhibitor III 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 382149 |
参考資料
| 参考資料の概要 |
|---|
| Ramiszewski, S.W., et al. 2002. J. Med. Chem. 45, 753. Jung, M., et al. 1999. J. Med. Chem. 42, 4669. |