400091 Sigma-AldrichHIF-Hydroxylase Inhibitor, DMOG - CAS 89464-63-1 - Calbiochem
HIF-Hydroxylase Inhibitor, DMOG, CAS 89464-63-1, is a cell-permeable 2-oxoglutarate (2-OG) analog that acts as a competitive inhibitor against all 2-OG-dependent dioxygenases.
More>> HIF-Hydroxylase Inhibitor, DMOG, CAS 89464-63-1, is a cell-permeable 2-oxoglutarate (2-OG) analog that acts as a competitive inhibitor against all 2-OG-dependent dioxygenases. Less<<同義語: N-(Methoxyoxoacetyl)-glycine methyl ester, Dimethyloxalylglycine, HIF Prolyl Hydroxylase Inhibitor I, HIF Aspartyl β-Hydroxylase Inhibitor, HIF Asparanginyl β-Hydroxylase Inhibitor
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
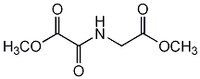
| 89464-63-1 | C₆H₉NO₅ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 400091-50MGCN |
|
ガラスビン | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 89464-63-1 |
| Form | Pink-white to peach-white |
| Hill Formula | C₆H₉NO₅ |
| Chemical formula | C₆H₉NO₅ |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Application | HIF-Hydroxylase Inhibitor, DMOG, CAS 89464-63-1, is a cell-permeable 2-oxoglutarate (2-OG) analog that acts as a competitive inhibitor against all 2-OG-dependent dioxygenases. |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 400091-50MGCN | 04055977212389 |
Documentation
HIF-Hydroxylase Inhibitor, DMOG - CAS 89464-63-1 - Calbiochem (M)SDS
| タイトル |
|---|
HIF-Hydroxylase Inhibitor, DMOG - CAS 89464-63-1 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 400091 |
参考資料
| 参考資料の概要 |
|---|
| Pollard, P.J., et al. 2008. Biochem. J. 416, 387. Chen, H., et al. 2006. Cancer Res. 66, 9009. Milkiewicz, M., et al. 2004. J. Physiol. 560, 21. Lando, D., et al. 2002. Science 295, 858. Jaakkola, P., et al. 2001. Science 292, 468. |