MFSL20SB3 MilliporeGamma sterilized Millipak® Final Fill 200 Filter Unit 5.0 µm 1/2 in. TC/TC
お勧めの製品
-

TUADP0695 Millipore テイクアップ アダプター Type E - 177 mm (6.95 in.) -

SN5MIX10EL0 Millipore MIX10 carrier -

MX0100 Millipore Maleic Acid - CAS 110-16-7 - Calbiochem -

ZF3000170 Milli-Q ZF3000170 -

5.08017.0001 Sigma-Aldrich nor-Binaltorphimine Dihydrochloride - CAS 105618-26-6 - Calbiochem -

1090981003 Supelco ヨウ素溶液 -

AB5776 Sigma-Aldrich Anti-Sox11 Antibody, pain -
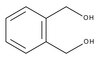
841297 Sigma-Aldrich 1,2-ベンゼンジメタノール -

MABE1921-25UL Sigma-Aldrich Anti-DDX3 Antibody, clone AO196 -

MABF2092-100UG Sigma-Aldrich Anti-CD44 Antibody, clone Hermes-1
概要
| Description | |
|---|---|
| Catalogue Number | MFSL20SB3 |
| Trade Name |
|
| Description | Gamma sterilized Millipak® Final Fill 200 Filter Unit 5.0 µm 1/2 in. TC/TC |
| Product Information | |
|---|---|
| HS Code | 8421 29 90 |
| Device Configuration | Gamma Gold Capsule |
| Connections, Inlet/Outlet | 13 mm (½ in.) stepped hose barb inlet and outlet |
| Maximum Differential Pressure, bar (psid) | Foward:80 psi (5.5 bar) at 25 °C (0.1 µm, 0.22 µm, 0.45 µm, hydrophobic 0.22 µm); Reverse: 10 psi (0.7 bar) at 25 °C |
| Maximum Inlet Pressure, bar (psi) | 80 psi (5.5 bar) at 25 °C |
| Good Manufacturing Practices | These products are manufactured in a facility which is certified to ISO® 9001:2015 Quality Management Systems. |
| Quality Level | MQ400 |
| Dimensions | |
|---|---|
| Filtration Area | 1000 cm² |
| Process Volume | 200 L |
| Inlet Connection Diameter | 1/2 in. |
| Outlet Connection Diameter | 1/2 in. |
| Materials Information | |
|---|---|
| Chemistry |
|
| Device Material |
|
| Support Material | Polysulfone |
| Packaging Information | |
|---|---|
| Material Size | 3 |
| Material Package | Double Easy-Open bag |
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| MFSL20SB3 | 04054839379543 |







