361566 Sigma-AldrichGSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
| 280744-09-4 | C₁₉H₁₂Cl₂N₂O₂ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 361566-10MGCN |
|
ガラスビン | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 280744-09-4 |
| Form | Orange solid |
| Hill Formula | C₁₉H₁₂Cl₂N₂O₂ |
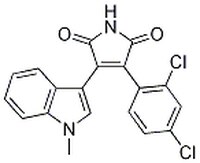
| Chemical formula | C₁₉H₁₂Cl₂N₂O₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 361566-10MGCN | 04055977214246 |
Documentation
GSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem (M)SDS
| タイトル |
|---|
GSK-3 Inhibitor IV, SB-216763 - CAS 280744-09-4 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 361566 |
参考資料
| 参考資料の概要 |
|---|
| Gross, E., et al. 2008. Am. J. Phy. Heart Circ Physiol. 294, H1497. Bain, J., et al. 2007. Biochem. J. 408, 297. Lu, D., et al. 2004. PNAS. 101, 3118. Carmichael, J., et al. 2002. J. Biol. Chem. 277, 33791. Culbert, A.A., et al. 2001. FEBS Lett. 507, 288. Lochhead, P.A., et al. 2001. Diabetes 50, 937. Cross, D.A., et al. 2001. J. Neurochem. 77, 94. Coghlan, M.P., et al. 2000. Chem. Biol. 7, 793. |