219555 Sigma-AldrichCathepsin K Substrate II, Fluorogenic - Calbiochem
An internally quenched, highly cathepsin K-selective, fluorogenic peptide substrate that is efficiently cleaved by CatK (kcat/Km = 0.426 µM⁻¹ S⁻¹), but not by Cathepsins B/L/S/F/H/V.
More>> An internally quenched, highly cathepsin K-selective, fluorogenic peptide substrate that is efficiently cleaved by CatK (kcat/Km = 0.426 µM⁻¹ S⁻¹), but not by Cathepsins B/L/S/F/H/V. Less<<同義語: Abz-HPG~GPQ-EDN₂ph
お勧めの製品
概要
| Replacement Information |
|---|
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 219555-1MGCN |
|
樹脂アンプル | 1 mg |
|
— |
| References | |
|---|---|
| References | Lecaille, F., et al. 2003. Biochem. J. 375, 307. |
| Product Information | |
|---|---|
| Form | Bright yellow solid |
| Formulation | Supplied as a trifluoroacetate salt. |
| Hill Formula | C₄₀H₅₀N₁₄O₁₂ |
| Chemical formula | C₄₀H₅₀N₁₄O₁₂ |
| Hygroscopic | Hygroscopic |
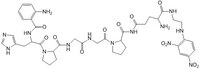
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Emission max. | |
| Excitation max. | |
| Peptide Sequence | Abz-His-Pro-Gly~Gly-Pro-Gln-EDDnp |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 219555-1MGCN | 04055977218114 |
Documentation
Cathepsin K Substrate II, Fluorogenic - Calbiochem (M)SDS
| タイトル |
|---|
Cathepsin K Substrate II, Fluorogenic - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 219555 |
参考資料
| 参考資料の概要 |
|---|
| Lecaille, F., et al. 2003. Biochem. J. 375, 307. |