264157 Sigma-AldrichCaspase Modulator I, 1541 - Calbiochem
同義語: Caspase-6 Inhibitor XI, Procaspase-3 Activator II, Procaspase-6 Activator I, 8-Methoxy-2-oxo-2H-chromene-3-carboxylic acid-(3-imidazo[1,2-a]pyridin-2-yl-phenyl)-amide, Caspase-3 Inhibitor XV
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| Empirical Formula |
|---|
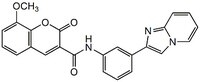
| C₂₄H₁₇N₃O₄ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 264157-10MGCN |
|
10 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable phenylimidazopyridinyl-methoxy coumarin compound that inhibits caspase-3 and caspase-6 activities (IC50 = 35 and 38 µM, respectively) by targeting both the large and small subunit active sites. Kinetic studies with 1541 and its 8-Hydroxy analog (Cat. No. 529663) indicate that at suboptimal inhibitor : proenzyme molar ratio (100:1 or less), the compound does not effectively occupies both active sites of and stabilizes the proenzyme in an “on-state” conformation that facilitates the subsequent autoproteolysis or autoactivation process via the unoccupied active site of procaspase-3 and -6 (EC50 = 2.4 and 2.8 µM, respectively). Shown to effectively induce apoptosis (up to 50 µM) in various cancer cell lines (IC50 ranges from 3.8 to 8.7 µM) in a caspase-3-dependent, but p53-, caspase-8-, and Bak-independent manner. Exhibits little inhibitory effect against active caspase-7 (30 nM) even at concentrations as high as 50 µM, nor does it activate procaspase-1 or -7 even after prolonged incubations upto 4 and 24 h, respectively. |
| Catalogue Number | 264157 |
| Brand Family | Calbiochem® |
| Synonyms | Caspase-6 Inhibitor XI, Procaspase-3 Activator II, Procaspase-6 Activator I, 8-Methoxy-2-oxo-2H-chromene-3-carboxylic acid-(3-imidazo[1,2-a]pyridin-2-yl-phenyl)-amide, Caspase-3 Inhibitor XV |
| References | |
|---|---|
| References | Wolan, D.W., et al. 2009. Science 326, 853. |
| Product Information | |
|---|---|
| Form | Yellow solid |
| Hill Formula | C₂₄H₁₇N₃O₄ |
| Chemical formula | C₂₄H₁₇N₃O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 264157-10MGCN | 04055977198485 |
Documentation
Caspase Modulator I, 1541 - Calbiochem (M)SDS
| タイトル |
|---|
Caspase Modulator I, 1541 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 264157 |
参考資料
| 参考資料の概要 |
|---|
| Wolan, D.W., et al. 2009. Science 326, 853. |