211200 Sigma-AldrichCAPE - CAS 104594-70-9 - Calbiochem
A cell-permeable active component of propolis from honeybee hives.
More>> A cell-permeable active component of propolis from honeybee hives. Less<<同義語: Caffeic Acid Phenethyl Ester, Synthetic
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
| 104594-70-9 | C₁₇H₁₆O₄ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 211200-25MGCN |
|
樹脂アンプル | 25 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 104594-70-9 |
| ATP Competitive | N |
| Form | White to off-white solid |
| Hill Formula | C₁₇H₁₆O₄ |
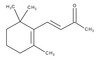
| Chemical formula | C₁₇H₁₆O₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HIV-integrase |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 211200-25MGCN | 07790788048525 |
Documentation
CAPE - CAS 104594-70-9 - Calbiochem (M)SDS
| タイトル |
|---|
CAPE - CAS 104594-70-9 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 211200 |
参考資料
| 参考資料の概要 |
|---|
| Nicklaus, M.C., et al. 1997. J. Med. Chem. 40, 920. Natarajan, K., et al. 1996. Proc. Natl. Acad. Sci. USA 93, 9090. Burke, T.R., Jr., et al. 1995. J. Med. Chem. 38, 4171. Laranjinha, J., et al. 1995. Arch. Biochem. Biophys. 323, 373. Su, Z.-Z., et al. 1995. Anticancer Res. 15, 1841. Zheng, Z.S., et al. 1995. Oncol. Res. 7, 445. Su, Z.-Z., et al. 1994. Cancer Res. 54, 1865. Fesen, M.R., et al. 1993. Proc. Natl. Acad. Sci. USA 90, 2399. Guarini, L., et al. 1992. Cell. Mol. Biol. 38, 513. |