196440 Sigma-AldrichBatimastat - CAS 130370-60-4 - Calbiochem
Batimastat, CAS 130370-60-4, is a potent inhibitor of a several metalloproteinases, including MMP-1, 2, 3, 7, 9, ΔMT1, ADAM8 & ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3 & 19 nM, respectively).
More>> Batimastat, CAS 130370-60-4, is a potent inhibitor of a several metalloproteinases, including MMP-1, 2, 3, 7, 9, ΔMT1, ADAM8 & ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3 & 19 nM, respectively). Less<<同義語: (4-N-Hydroxyamino)-2R-isobutyl-3S-(thienylthiomethyl)succinyl)-L-phenylalanine-N-methylamide, BB-94
お勧めの製品
概要
| Replacement Information |
|---|
主要スペック表
| CAS # | Empirical Formula |
|---|---|
| 130370-60-4 | C₂₃H₃₁N₃O₄S₂ |
価格&在庫状況
| カタログ番号 | 在庫状況 | 包装 | Qty/Pk | 価格 | 数量 | |
|---|---|---|---|---|---|---|
| 196440-5MGCN |
|
ガラスビン | 5 mg |
|
— |
| Description | |
|---|---|
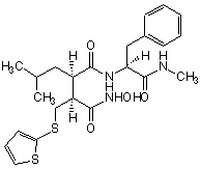
| Overview | A Marimastat (Cat. No. 444289) type of peptidyl hydroxamate-based inhibitor that potently inhibits a broad-spectrum of metalloproteinases, including MMP-1, MMP-2, MMP-3/stromelysin, MMP-7/matrilysin, MMP-9, ΔMT1 (MMP-14 without TM domain), ADAM8, and ADAM17/TACE (IC50 = 3, 4, 20, 6, 4, 2.08, 51.3, and 19 nM, respectively), by targeting both the substrate binding site and the active-site Zn2+, while being much less potent toward ACE (Angiotensin Converting Enzyme) or α-secretase (IC50 = 1.6 and 3.3 µM, respectively). Batimastat is widely used in studying the involvement of MMPs in cancinogenesis and non-cancer pathological processes both in cultures in vitro and in animals in vivo. Also available as a 25 mM solution in DMSO (Cat. No. 508408). |
| Catalogue Number | 196440 |
| Brand Family | Calbiochem® |
| Synonyms | (4-N-Hydroxyamino)-2R-isobutyl-3S-(thienylthiomethyl)succinyl)-L-phenylalanine-N-methylamide, BB-94 |
| Product Information | |
|---|---|
| CAS number | 130370-60-4 |
| Form | Off-white solid |
| Hill Formula | C₂₃H₃₁N₃O₄S₂ |
| Chemical formula | C₂₃H₃₁N₃O₄S₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| カタログ番号 | GTIN |
| 196440-5MGCN | 04055977206500 |
Documentation
Batimastat - CAS 130370-60-4 - Calbiochem (M)SDS
| タイトル |
|---|
Batimastat - CAS 130370-60-4 - Calbiochem 試験成績書(CoA)
| タイトル | ロット番号 |
|---|---|
| 196440 |
参考資料
| 参考資料の概要 |
|---|
| Schlomann, U., et al. 2002. J. Biol. Chem. 277, 48210. Whittaker, M., et al. 1999. Chem. Rev. 99, 2735. Parvathy, S., et al. 1998. Biochemistry 37, 1680. Parvathy, S., et al. 1998. FEBS Lett. 431, 63. Yamamoto, M., et al. 1998. J. Med. Chem. 41, 1209. Moss, M.L., et al. 1997. Nature 385, 733. Eccles, S.A., et al. 1996. Cancer Res. 56, 2815. Brown, P.D. 1995. Advan. Enzyme Regul. 35, 293. Wang, X., et al. 1994. Cancer Res. 54, 4726. Davies, B., et al. 1993. Cancer Res. 53, 2087. |