Automatic Integrity Test Instrument Qualification
Millipore validation engineers, familiar with the operation, can provide on-site assistance with performing Installation Qualification (IQ) and Operational Qualification (OQ).
もっと見る
Millipore validation engineers, familiar with the operation, can provide on-site assistance with performing Installation Qualification (IQ) and Operational Qualification (OQ). 表示を少なく
More>>
Less<<

お勧めの製品
-

MABE1064-25UG Sigma-Aldrich Anti-HsSAS-6 Antibody, clone 91.390.21 -

14-610M Sigma-Aldrich Flt-3 (D835Y) Protein, active, 250 µg -
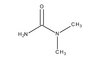
820509 Sigma-Aldrich N,N-ジメチル尿素 -

CR0372006 Millipore ポリガード(Polygard)-CR カートリッジ フィルター 20 in. 3.0 µm コード7 シリコン -

843845 Sigma-Aldrich 二硫化セレン -

HTS013RTA Sigma-Aldrich Ready-to-Assay™ CCR8 Chemokine Family Receptor Frozen Cells -

AG316 Sigma-Aldrich Acetylcholine Receptor-ε, control peptide for AB5938 -

8182030025 Sigma-Aldrich クロロギ酸9-フルオレニルメチル -

435801 Sigma-Aldrich Licofelone, Potassium Salt, Monohydrate - CAS 156897-06-2 - Calbiochem -

1084311003 Supelco チトリプレックス™ III 溶液
概要
仕様
ご注文情報
Documentation
関連製品&アプリケーション
製品ファミリー

Integritest 4シリーズ 自動フィルター完全性試験機Easy-to-use, portable, fully automated integrity test system詳細はこちら >> |
関連製品: Application Facete
| Disinfection control |
| Dialysis and Filtration |
カテゴリー
| Biopharmaceutical Manufacturing > Downstream Processing > Sterile Filtration > Integrity Testing |
Service Description
All of our Provantage® services include a team of experienced engineers who are personally committed to ensuring your processing success and will partner with you to overcome any challenge. This service includes qualification of filters used in your production process under worst-case conditions. It also includes application support, administrator training and assistance for performance qualification (PQ). Our Absolute Qualification Service is intended for those who are working in heavily regulated environments following cGMP guidelines.
Qualification Protocol
The protocols provided by us are designed to specific GMP guidelines and when executed by a qualified person, provide the user with results that satisfy the requirements in the guidelines.
Installation and Operational Qualification
The qualification execution includes labor (one engineer for 2 days) to execute the IQOQ protocol in the environment in which the instrument will be used. Our engineers are skilled in the operation of the equipment and provide specific instruments calibrated against national reference. After completion of the validation exercise, the data is collected and a ready to approve documentation package is provided.
Custom Service Agreements
We offer a range of qualification and preventive maintenance services to meet your unique manufacturing requirements resulting in peace of mind and maximum operational flexibility. The Integritest® 4 service agreements include inspection, calibration, maintenance and repair.
Corrective Maintenance Support
For quick resolution of any performance issues that may arise, our certified field service engineers are available to ensure that your Integritest® 4 system is up and running at optimum performance with minimum delay.
Preventive Maintenance Support
Using preventive maintenance protocols, your field service engineer will replace worn components and verify your Integritest® 4 system to ensure trouble-free operation.
How to Request Integritest® 4 System qualification protocol and services
To request on-site qualification or to get information on other validation services, call your Application Specialist or the office nearest you.






