A genome-wide RNAi screen identifies proteins modulating aberrant FLT3-ITD signaling.
Caldarelli, A; Müller, JP; Paskowski-Rogacz, M; Herrmann, K; Bauer, R; Koch, S; Heninger, AK; Krastev, D; Ding, L; Kasper, S; Fischer, T; Brodhun, M; Böhmer, FD; Buchholz, F
Leukemia
27
2301-10
2013
概要を表示する
Fms-like tyrosine kinase-3 is a commonly mutated gene in acute myeloid leukemia, with about one-third of patients carrying an internal-tandem duplication of the juxtamembrane domain in the receptor (FLT3-ITD). FLT3-ITD exhibits altered signaling quality, including aberrant activation of STAT5. To identify genes affecting FLT3-ITD-mediated STAT5 signaling, we performed an esiRNA-based RNAi screen utilizing a STAT5-driven reporter assay. Knockdowns that caused reduced FLT3-ITD-mediated STAT5 signaling were enriched for genes encoding proteins involved in protein secretion and intracellular protein transport, indicating that modulation of protein transport processes could potentially be used to reduce constitutive STAT5 signaling in FLT3-ITD-positive cells. The relevance of KDELR1, a component involved in the Golgi-ER retrograde transport, was further analyzed. In FLT3-ITD-expressing leukemic MV4-11 cells, downregulation of KDELR1 resulted in reduced STAT5 activation, proliferation and colony-forming capacity. Stable shRNA-mediated depletion of KDELR1 in FLT3-ITD-expressing 32D cells likewise resulted in reduced STAT5 signaling and cell proliferation. Importantly, these cells also showed a reduced capacity to generate a leukemia-like disease in syngeneic C3H/HeJ mice. Together our data suggest intracellular protein transport as a potential target for FLT3-ITD driven leukemias, with KDELR1 emerging as a positive modulator of oncogenic FLT3-ITD activity. | | 23508117
 |
The regulation of Rasd1 expression by glucocorticoids and prolactin controls peripartum maternal insulin secretion.
Lellis-Santos, C; Sakamoto, LH; Bromati, CR; Nogueira, TC; Leite, AR; Yamanaka, TS; Kinote, A; Anhê, GF; Bordin, S
Endocrinology
153
3668-78
2012
概要を表示する
The transition from gestation to lactation is characterized by a robust adaptation of maternal pancreatic β-cells. Consistent with the loss of β-cell mass, glucose-induced insulin secretion is down-regulated in the islets of early lactating dams. Extensive experimental evidence has demonstrated that the surge of prolactin is responsible for the morphofunctional remodeling of the maternal endocrine pancreas during pregnancy, but the precise molecular mechanisms by which this phenotype is rapidly reversed after delivery are not completely understood. This study investigated whether glucocorticoid-regulated expression of Rasd1/Dexras, a small inhibitory G protein, is involved in this physiological plasticity. Immunofluorescent staining demonstrated that Rasd1 is localized within pancreatic β-cells. Rasd1 expression in insulin-secreting cells was increased by dexamethasone and decreased by prolactin. In vivo data confirmed that Rasd1 expression is decreased in islets from pregnant rats and increased in islets from lactating mothers. Knockdown of Rasd1 abolished the inhibitory effects of dexamethasone on insulin secretion and the protein kinase A, protein kinase C, and ERK1/2 pathways. Chromatin immunoprecipitation experiments revealed that glucocorticoid receptor (GR) and signal transducer and activator of transcription 5b (STAT5b) cooperatively mediate glucocorticoid-induced Rasd1 expression in islets. Prolactin inhibited the stimulatory effect of GR/STAT5b complex on Rasd1 transcription. Overall, our data indicate that the stimulation of Rasd1 expression by glucocorticoid at the end of pregnancy reverses the increased insulin secretion that occurs during pregnancy. Prolactin negatively regulates this pathway by inhibiting GR/STAT5b transcriptional activity on the Rasd1 gene. | | 22700767
 |
Transcriptional profiling of bipotential embryonic liver cells to identify liver progenitor cell surface markers.
Ochsner, SA; Strick-Marchand, H; Qiu, Q; Venable, S; Dean, A; Wilde, M; Weiss, MC; Darlington, GJ
Stem cells (Dayton, Ohio)
25
2476-87
2007
概要を表示する
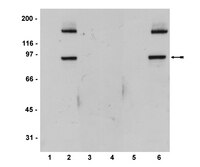
The ability to purify to homogeneity a population of hepatic progenitor cells from adult liver is critical for their characterization prior to any therapeutic application. As a step in this direction, we have used a bipotential liver cell line from 14 days postcoitum mouse embryonic liver to compile a list of cell surface markers expressed specifically by liver progenitor cells. These cells, known as bipotential mouse embryonic liver (BMEL) cells, proliferate in an undifferentiated state and are capable of differentiating into hepatocyte-like and cholangiocyte-like cells in vitro. Upon transplantation, BMEL cells are capable of differentiating into hepatocytes and cholangiocytes in vivo. Microarray and Gene Ontology (GO) analysis of gene expression in the 9A1 and 14B3 BMEL cell lines grown under proliferating and differentiating conditions was used to identify cell surface markers preferentially expressed in the bipotential undifferentiated state. This analysis revealed that proliferating BMEL cells express many genes involved in cell cycle regulation, whereas differentiation of BMEL cells by cell aggregation causes a switch in gene expression to functions characteristic of mature hepatocytes. In addition, microarray data and protein analysis indicated that the Notch signaling pathway could be involved in maintaining BMEL cells in an undifferentiated stem cell state. Using GO annotation, a list of cell surface markers preferentially expressed on undifferentiated BMEL cells was generated. One marker, Cd24a, is specifically expressed on progenitor oval cells in livers of diethyl 1,4-dihydro-2,4,6-trimethyl-3,5-pyridinedicarboxylate-treated animals. We therefore consider Cd24a expression a candidate molecule for purification of hepatic progenitor cells. Disclosure of potential conflicts of interest is found at the end of this article. | Western Blotting | 17641245
 |
Altered interleukin-12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1.
Gollob, J A, et al.
Blood, 91: 1341-54 (1998)
1998
概要を表示する
T-cell activation in response to interleukin-12 (IL-12) is mediated through signaling events that include the tyrosine phosphorylation of STAT4. IL-12 responsiveness and the ability of IL-12 to activate STAT4 is different in T cells induced to differentiate into a Th1 or Th2 phenotype. In this report, we show that STAT5, STAT1alpha, and STAT1beta, in addition to STAT4, are tyrosine phosphorylated in response to IL-12 in phytohemagglutinin (PHA)-activated human T cells. To understand how the activation of these STATs contributes to T-cell IL-12 responsiveness, we analyzed the IL-12-induced activation of STAT5 and STAT1 in T cells stimulated to undergo Th1 or Th2 differentiation. The IL-12-induced tyrosine phosphorylation of STAT5 and STAT1, but not STAT4, is augmented in T cells activated into Th1 cells with PHA + interferon-gamma (IFN-gamma) compared with T cells activated with PHA alone. STAT5 DNA binding induced by IL-12 is also augmented in T cells activated with PHA + IFN-gamma compared with T cells activated with PHA alone, whereas STAT4 DNA binding is not increased. In contrast, the IL-12-induced activation of these STATs is inhibited in T cells activated into Th2 cells with PHA + IL-4. The enhancement of IL-12 signaling by IFN-gamma is not a direct effect of IFN-gamma on T cells, but rather is mediated by IL-12 that is produced by antigen-presenting cells in response to IFN-gamma. This positive autoregulatory effect of IL-12 on the activation of select STATs correlates with an increase in T-cell IFN-gamma production in response to IL-12. These findings suggest that the activation of STAT5 and STAT1 may augment select STAT4-dependent functional responses to IL-12 in Th1 cells. | | 9454765
 |