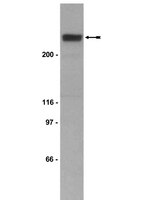
Mechanistic analysis of the role of bromodomain-containing protein 4 (BRD4) in BRD4-NUT oncoprotein-induced transcriptional activation.
Wang, R; You, J
The Journal of biological chemistry
290
2744-58
2015
概要を表示する
NUT midline carcinoma (NMC) is a rare but highly aggressive cancer typically caused by the translocation t(15;19), which results in the formation of the BRD4-NUT fusion oncoprotein. Previous studies have demonstrated that fusion of the NUT protein with the double bromodomains of BRD4 may significantly alter the cellular gene expression profile to contribute to NMC tumorigenesis. However, the mechanistic details of this BRD4-NUT function remain poorly understood. In this study, we examined the NUT function in transcriptional regulation by targeting it to a LacO transgene array integrated in U2OS 2-6-3 cells, which allow us to visualize how NUT alters the in situ gene transcription dynamic. Using this system, we demonstrated that the NUT protein tethered to the LacO locus recruits p300/CREB-binding protein (CBP), induces histone hyperacetylation, and enriches BRD4 to the transgene array chromatin foci. We also discovered that, in BRD4-NUT expressed in NMC cells, the NUT moiety of the fusion protein anchored to chromatin by the double bromodomains also stimulates histone hyperacetylation, which causes BRD4 to bind tighter to chromatin. Consequently, multiple BRD4-interacting factors are recruited to the NUT-associated chromatin locus to activate in situ transgene expression. This gene transcription function was repressed by either expression of a dominant negative inhibitor of the p300-NUT interaction or treatment with (+)-JQ1, which dissociates BRD4 from the LacO chromatin locus. Our data support a model in which BRD4-NUT-stimulated histone hyperacetylation recruits additional BRD4 and interacting partners to support transcriptional activation, which underlies the BRD4-NUT oncogenic mechanism in NMC. | Immunofluorescence | | 25512383
 |
MAF1 represses CDKN1A through a Pol III-dependent mechanism.
Lee, YL; Li, YC; Su, CH; Chiao, CH; Lin, IH; Hsu, MT
eLife
4
e06283
2015
概要を表示する
MAF1 represses Pol III-mediated transcription by interfering with TFIIIB and Pol III. Herein, we found that MAF1 knockdown induced CDKN1A transcription and chromatin looping concurrently with Pol III recruitment. Simultaneous knockdown of MAF1 with Pol III or BRF1 (subunit of TFIIIB) diminished the activation and looping effect, which indicates that recruiting Pol III was required for activation of Pol II-mediated transcription and chromatin looping. Chromatin-immunoprecipitation analysis after MAF1 knockdown indicated enhanced binding of Pol III and BRF1, as well as of CFP1, p300, and PCAF, which are factors that mediate active histone marks, along with the binding of TATA binding protein (TBP) and POLR2E to the CDKN1A promoter. Simultaneous knockdown with Pol III abolished these regulatory events. Similar results were obtained for GDF15. Our results reveal a novel mechanism by which MAF1 and Pol III regulate the activity of a protein-coding gene transcribed by Pol II. | | | 26067234
 |
DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression.
Cho, MH; Park, JH; Choi, HJ; Park, MK; Won, HY; Park, YJ; Lee, CH; Oh, SH; Song, YS; Kim, HS; Oh, YH; Lee, JY; Kong, G
Nature communications
6
7821
2015
概要を表示する
DOT1L has emerged as an anticancer target for MLL-associated leukaemias; however, its functional role in solid tumours is largely unknown. Here we identify that DOT1L cooperates with c-Myc and p300 acetyltransferase to epigenetically activate epithelial-mesenchymal transition (EMT) regulators in breast cancer progression. DOT1L recognizes SNAIL, ZEB1 and ZEB2 promoters via interacting with the c-Myc-p300 complex and facilitates lysine-79 methylation and acetylation towards histone H3, leading to the dissociation of HDAC1 and DNMT1 in the regions. The upregulation of these EMT regulators by the DOT1L-c-Myc-p300 complex enhances EMT-induced breast cancer stem cell (CSC)-like properties. Furthermore, in vivo orthotopic xenograft models show that DOT1L is required for malignant transformation of breast epithelial cells and breast tumour initiation and metastasis. Clinically, DOT1L expression is associated with poorer survival and aggressiveness of breast cancers. Collectively, we suggest that cooperative effect of DOT1L and c-Myc-p300 is critical for acquisition of aggressive phenotype of breast cancer by promoting EMT/CSC. | | | 26199140
 |
Src is activated by the nuclear receptor peroxisome proliferator-activated receptor β/δ in ultraviolet radiation-induced skin cancer.
Montagner, A; Delgado, MB; Tallichet-Blanc, C; Chan, JS; Sng, MK; Mottaz, H; Degueurce, G; Lippi, Y; Moret, C; Baruchet, M; Antsiferova, M; Werner, S; Hohl, D; Saati, TA; Farmer, PJ; Tan, NS; Michalik, L; Wahli, W
EMBO molecular medicine
6
80-98
2014
概要を表示する
Although non-melanoma skin cancer (NMSC) is the most common human cancer and its incidence continues to rise worldwide, the mechanisms underlying its development remain incompletely understood. Here, we unveil a cascade of events involving peroxisome proliferator-activated receptor (PPAR) β/δ and the oncogene Src, which promotes the development of ultraviolet (UV)-induced skin cancer in mice. UV-induced PPARβ/δ activity, which directly stimulated Src expression, increased Src kinase activity and enhanced the EGFR/Erk1/2 signalling pathway, resulting in increased epithelial-to-mesenchymal transition (EMT) marker expression. Consistent with these observations, PPARβ/δ-null mice developed fewer and smaller skin tumours, and a PPARβ/δ antagonist prevented UV-dependent Src stimulation. Furthermore, the expression of PPARβ/δ positively correlated with the expression of SRC and EMT markers in human skin squamous cell carcinoma (SCC), and critically, linear models applied to several human epithelial cancers revealed an interaction between PPARβ/δ and SRC and TGFβ1 transcriptional levels. Taken together, these observations motivate the future evaluation of PPARβ/δ modulators to attenuate the development of several epithelial cancers. | Western Blotting | | 24203162
 |
An H2A histone isotype regulates estrogen receptor target genes by mediating enhancer-promoter-3'-UTR interactions in breast cancer cells.
Su, CH; Tzeng, TY; Cheng, C; Hsu, MT
Nucleic acids research
42
3073-88
2014
概要を表示する
A replication-dependent histone H2A isotype, H2ac, is upregulated in MCF-7 cells and in estrogen receptor-positive clinical breast cancer tissues. Cellular depletion of this H2A isotype leads to defective estrogen signaling, loss of cell proliferation and cell cycle arrest at G0/G1 phase. H2ac mediates regulation of estrogen receptor target genes, particularly BCL2 and c-MYC, by recruiting estrogen receptor alpha through its HAR domain and facilitating the formation of a chromatin loop between the promoter, enhancer and 3'-untranslated region of the respective genes. These findings reveal a new role for histone isotypes in the regulation of gene expression in cancer cells, and suggest that these molecules may be targeted for anti-cancer drug discovery. | | | 24371278
 |
Otx2 and Oct4 drive early enhancer activation during embryonic stem cell transition from naive pluripotency.
Yang, SH; Kalkan, T; Morissroe, C; Marks, H; Stunnenberg, H; Smith, A; Sharrocks, AD
Cell reports
7
1968-81
2014
概要を表示する
Embryonic stem cells (ESCs) are unique in that they have the capacity to differentiate into all of the cell types in the body. We know a lot about the complex transcriptional control circuits that maintain the naive pluripotent state under self-renewing conditions but comparatively less about how cells exit from this state in response to differentiation stimuli. Here, we examined the role of Otx2 in this process in mouse ESCs and demonstrate that it plays a leading role in remodeling the gene regulatory networks as cells exit from ground state pluripotency. Otx2 drives enhancer activation through affecting chromatin marks and the activity of associated genes. Mechanistically, Oct4 is required for Otx2 expression, and reciprocally, Otx2 is required for efficient Oct4 recruitment to many enhancer regions. Therefore, the Oct4-Otx2 regulatory axis actively establishes a new regulatory chromatin landscape during the early events that accompany exit from ground state pluripotency. | | | 24931607
 |
An amino terminal phosphorylation motif regulates intranuclear compartmentalization of Olig2 in neural progenitor cells.
Meijer, DH; Sun, Y; Liu, T; Kane, MF; Alberta, JA; Adelmant, G; Kupp, R; Marto, JA; Rowitch, DH; Nakatani, Y; Stiles, CD; Mehta, S
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
8507-18
2014
概要を表示する
The bHLH transcription factor Olig2 is expressed in cycling neural progenitor cells but also in terminally differentiated, myelinating oligodendrocytes. Sustained expression of Olig2 is counterintuitive because all known functions of the protein in expansion of neural progenitors and specification of oligodendrocyte progenitors are completed with the formation of mature white matter. How are the biological functions of Olig2 suppressed in terminally differentiated oligodendrocytes? In previous studies, we have shown that a triple serine motif in the amino terminus of Olig2 is phosphorylated in cycling neural progenitors but not in their differentiated progeny. We now show that phosphorylation of the triple serine motif regulates intranuclear compartmentalization of murine Olig2. Phosphorylated Olig2 is preferentially localized to a transcriptionally active "open" chromatin compartment together with coregulator proteins essential for regulation of gene expression. Unphosphorylated Olig2, as seen in mature white matter, is localized mainly within a transcriptionally inactive, chromatin fraction characterized by condensed and inaccessible DNA. Of special note is the observation that the p53 tumor suppressor protein is confined to the open chromatin fraction. Proximity ligation assays show that phosphorylation brings Olig2 within 30 nm of p53 within the open chromatin compartment. The data thus shed light on previously noted promitogenic functions of phosphorylated Olig2, which reflect, at least in part, an oppositional relationship with p53 functions. | Western Blotting | | 24948806
 |
Involvement of p300/CBP and epigenetic histone acetylation in TGF-β1-mediated gene transcription in mesangial cells.
Yuan, H; Reddy, MA; Sun, G; Lanting, L; Wang, M; Kato, M; Natarajan, R
American journal of physiology. Renal physiology
304
F601-13
2013
概要を表示する
Transforming growth factor-β1 (TGF-β1)-induced expression of plasminogen activator inhibitor-1 (PAI-1) and p21 in renal mesangial cells (MCs) plays a major role in glomerulosclerosis and hypertrophy, key events in the pathogenesis of diabetic nephropathy. However, the involvement of histone acetyl transferases (HATs) and histone deacetylases (HDACs) that regulate epigenetic histone lysine acetylation, and their interaction with TGF-β1-responsive transcription factors, are not clear. We evaluated the roles of histone acetylation, specific HATs, and HDACs in TGF-β1-induced gene expression in rat mesangial cells (RMCs) and in glomeruli from diabetic mice. Overexpression of HATs CREB binding protein (CBP) or p300, but not p300/CBP-activating factor, significantly enhanced TGF-β1-induced PAI-1 and p21 mRNA levels as well as transactivation of their promoters in RMCs. Conversely, they were significantly attenuated by HAT domain mutants of CBP and p300 or overexpression of HDAC-1 and HDAC-5. Chromatin immunoprecipitation assays showed that TGF-β1 treatment led to a time-dependent enrichment of histone H3-lysine9/14-acetylation (H3K9/14Ac) and p300/CBP occupancies around Smad and Sp1 binding sites at the PAI-1 and p21 promoters. TGF-β1 also enhanced the interaction of p300 with Smad2/3 and Sp1 and increased Smad2/3 acetylation. High glucose-treated RMCs exhibited increased PAI-1 and p21 levels, and promoter H3K9/14Ac, which were blocked by TGF-β1 antibodies. Furthermore, increased PAI-1 and p21 expression was associated with elevated promoter H3K9/14Ac levels in glomeruli from diabetic mice. Thus TGF-β1-induced PAI-1 and p21 expression involves interaction of p300/CBP with Smads and Sp1, and increased promoter access via p300/CBP-induced H3K9/14Ac. This in turn can augment glomerular dysfunction linked to diabetic nephropathy. | | | 23235480
 |
Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression.
Dangi-Garimella, S; Sahai, V; Ebine, K; Kumar, K; Munshi, HG
PloS one
8
e64566
2013
概要を表示する
Pancreatic ductal adenocarcinoma (PDAC) is associated with a pronounced collagen-rich stromal reaction that has been shown to contribute to chemo-resistance. We have previously shown that PDAC cells are resistant to gemcitabine chemotherapy in the collagen microenvironment because of increased expression of the chromatin remodeling protein high mobility group A2 (HMGA2). We have now found that human PDAC tumors display higher levels of histone H3K9 and H3K27 acetylation in fibrotic regions. We show that relative to cells grown on tissue culture plastic, PDAC cells grown in three-dimensional collagen gels demonstrate increased histone H3K9 and H3K27 acetylation, along with increased expression of p300, PCAF and GCN5 histone acetyltransferases (HATs). Knocking down HMGA2 attenuates the effect of collagen on histone H3K9 and H3K27 acetylation and on collagen-induced p300, PCAF and GCN5 expression. We also show that human PDAC tumors with HMGA2 demonstrate increased histone H3K9 and H3K27 acetylation. Additionally, we show that cells in three-dimensional collagen gels demonstrate increased protection against gemcitabine. Significantly, down-regulation of HMGA2 or p300, PCAF and GCN5 HATs sensitizes the cells to gemcitabine in three-dimensional collagen. Overall, our results increase our understanding of how the collagen microenvironment contributes to chemo-resistance in vitro and identify HATs as potential therapeutic targets against this deadly cancer. | | | 23696899
 |
Up-regulation of ciliary neurotrophic factor in astrocytes by aspirin: implications for remyelination in multiple sclerosis.
Modi, KK; Sendtner, M; Pahan, K
J Biol Chem
288
18533-45
2013
概要を表示する
Ciliary neurotrophic factor (CNTF) is a promyelinating trophic factor, and the mechanisms by which CNTF expression could be increased in the brain are poorly understood. Acetylsalicylic acid (aspirin) is one of the most widely used analgesics. Interestingly, aspirin increased mRNA and protein expression of CNTF in primary mouse and human astrocytes in a dose- and time-dependent manner. Aspirin induced the activation of protein kinase A (PKA) but not protein kinase C (PKC). H-89, an inhibitor of PKA, abrogated aspirin-induced expression of CNTF. The activation of cAMP-response element-binding protein (CREB), but not NF-κB, by aspirin, the abrogation of aspirin-induced expression of CNTF by siRNA knockdown of CREB, the presence of a consensus cAMP-response element in the promoter of CNTF, and the recruitment of CREB and CREB-binding protein to the CNTF promoter by aspirin suggest that aspirin increases the expression of the Cntf gene via the activation of CREB. Furthermore, we demonstrate that aspirin-induced astroglial CNTF was also functionally active and that supernatants of aspirin-treated astrocytes of wild type, but not Cntf null, mice increased myelin-associated proteins in oligodendrocytes and protected oligodendrocytes from TNF-α insult. These results highlight a new and novel myelinogenic property of aspirin, which may be of benefit for multiple sclerosis and other demyelinating disorders. | | | 23653362
 |