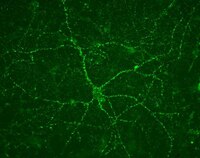
Experimental microembolism induces localized neuritic pathology in guinea pig cerebrum.
Li, JM; Cai, Y; Liu, F; Yang, L; Hu, X; Patrylo, PR; Cai, H; Luo, XG; Xiao, D; Yan, XX
Oncotarget
6
10772-85
2015
概要を表示する
Microbleeds are a common finding in aged human brains. In Alzheimer's disease (AD), neuritic plaques composed of β-amyloid (Aβ) deposits and dystrophic neurites occur frequently around cerebral vasculature, raising a compelling question as to whether, and if so, how, microvascular abnormality and amyloid/neuritic pathology might be causally related. Here we used a guinea pig model of cerebral microembolism to explore a potential inductive effect of vascular injury on neuritic and amyloid pathogenesis. Brains were examined 7-30 days after experimental microvascular embolization occupying ~0.5% of total cortical area. Compared to sham-operated controls, glial fibrillary acidic protein immunoreactivity was increased in the embolized cerebrum, evidently around intracortical vasculature. Swollen/sprouting neurites exhibiting increased reactivity of nicotinamide adenine dinucleotide phosphate diaphorase, parvalbumin, vesicular glutamate transporter 1 and choline acetyltransferase appeared locally in the embolized brains in proximity to intracortical vasculature. The embolization-induced swollen/sprouting neurites were also robustly immunoreactive for β-amyloid precursor protein and β-secretase-1, the substrate and initiating enzyme for Aβ genesis. These experimental data suggest that microvascular injury can induce multisystem neuritic pathology associated with an enhanced amyloidogenic potential in wild-type mammalian brain. | | | 25871402
 |
Selective conversion of fibroblasts into peripheral sensory neurons.
Blanchard, JW; Eade, KT; Szűcs, A; Lo Sardo, V; Tsunemoto, RK; Williams, D; Sanna, PP; Baldwin, KK
Nature neuroscience
18
25-35
2015
概要を表示する
Humans and mice detect pain, itch, temperature, pressure, stretch and limb position via signaling from peripheral sensory neurons. These neurons are divided into three functional classes (nociceptors/pruritoceptors, mechanoreceptors and proprioceptors) that are distinguished by their selective expression of TrkA, TrkB or TrkC receptors, respectively. We found that transiently coexpressing Brn3a with either Ngn1 or Ngn2 selectively reprogrammed human and mouse fibroblasts to acquire key properties of these three classes of sensory neurons. These induced sensory neurons (iSNs) were electrically active, exhibited distinct sensory neuron morphologies and matched the characteristic gene expression patterns of endogenous sensory neurons, including selective expression of Trk receptors. In addition, we found that calcium-imaging assays could identify subsets of iSNs that selectively responded to diverse ligands known to activate itch- and pain-sensing neurons. These results offer a simple and rapid means for producing genetically diverse human sensory neurons suitable for drug screening and mechanistic studies. | | | 25420069
 |
Cell autonomous regulation of hippocampal circuitry via Aph1b-γ-secretase/neuregulin 1 signalling.
Fazzari, P; Snellinx, A; Sabanov, V; Ahmed, T; Serneels, L; Gartner, A; Shariati, SA; Balschun, D; De Strooper, B
eLife
3
2014
概要を表示する
Neuregulin 1 (NRG1) and the γ-secretase subunit APH1B have been previously implicated as genetic risk factors for schizophrenia and schizophrenia relevant deficits have been observed in rodent models with loss of function mutations in either gene. Here we show that the Aph1b-γ-secretase is selectively involved in Nrg1 intracellular signalling. We found that Aph1b-deficient mice display a decrease in excitatory synaptic markers. Electrophysiological recordings show that Aph1b is required for excitatory synaptic transmission and plasticity. Furthermore, gain and loss of function and genetic rescue experiments indicate that Nrg1 intracellular signalling promotes dendritic spine formation downstream of Aph1b-γ-secretase in vitro and in vivo. In conclusion, our study sheds light on the physiological role of Aph1b-γ-secretase in brain and provides a new mechanistic perspective on the relevance of NRG1 processing in schizophrenia. | Immunohistochemistry | Mouse | 24891237
 |
Molecular and functional definition of the developing human striatum.
Onorati, M; Castiglioni, V; Biasci, D; Cesana, E; Menon, R; Vuono, R; Talpo, F; Laguna Goya, R; Lyons, PA; Bulfamante, GP; Muzio, L; Martino, G; Toselli, M; Farina, C; Barker, RA; Biella, G; Cattaneo, E
Nature neuroscience
1804-15
2014
概要を表示する
The complexity of the human brain derives from the intricate interplay of molecular instructions during development. Here we systematically investigated gene expression changes in the prenatal human striatum and cerebral cortex during development from post-conception weeks 2 to 20. We identified tissue-specific gene coexpression networks, differentially expressed genes and a minimal set of bimodal genes, including those encoding transcription factors, that distinguished striatal from neocortical identities. Unexpected differences from mouse striatal development were discovered. We monitored 36 determinants at the protein level, revealing regional domains of expression and their refinement, during striatal development. We electrophysiologically profiled human striatal neurons differentiated in vitro and determined their refined molecular and functional properties. These results provide a resource and opportunity to gain global understanding of how transcriptional and functional processes converge to specify human striatal and neocortical neurons during development. | Immunofluorescence | | 25383901
 |
Cortical region-specific engraftment of embryonic stem cell-derived neural progenitor cells restores axonal sprouting to a subcortical target and achieves motor functional recovery in a mouse model of neonatal hypoxic-ischemic brain injury.
Shinoyama, M; Ideguchi, M; Kida, H; Kajiwara, K; Kagawa, Y; Maeda, Y; Nomura, S; Suzuki, M
Frontiers in cellular neuroscience
7
128
2013
概要を表示する
Hypoxic-ischemic encephalopathy (HIE) at birth could cause cerebral palsy (CP), mental retardation, and epilepsy, which last throughout the individual's lifetime. However, few restorative treatments for ischemic tissue are currently available. Cell replacement therapy offers the potential to rescue brain damage caused by HI and to restore motor function. In the present study, we evaluated the ability of embryonic stem cell-derived neural progenitor cells (ES-NPCs) to become cortical deep layer neurons, to restore the neural network, and to repair brain damage in an HIE mouse model. ES cells stably expressing the reporter gene GFP are induced to a neural precursor state by stromal cell co-culture. Forty-hours after the induction of HIE, animals were grafted with ES-NPCs targeting the deep layer of the motor cortex in the ischemic brain. Motor function was evaluated 3 weeks after transplantation. Immunohistochemistry and neuroanatomical tracing with GFP were used to analyze neuronal differentiation and axonal sprouting. ES-NPCs could differentiate to cortical neurons with pyramidal morphology and expressed the deep layer-specific marker, Ctip2. The graft showed good survival and an appropriate innervation pattern via axonal sprouting from engrafted cells in the ischemic brain. The motor functions of the transplanted HIE mice also improved significantly compared to the sham-transplanted group. These findings suggest that cortical region specific engraftment of preconditioned cortical precursor cells could support motor functional recovery in the HIE model. It is not clear whether this is a direct effect of the engrafted cells or due to neurotrophic factors produced by these cells. These results suggest that cortical region-specific NPC engraftment is a promising therapeutic approach for brain repair. | | | 23970853
 |
Afadin is required for maintenance of dendritic structure and excitatory tone.
Srivastava, DP; Copits, BA; Xie, Z; Huda, R; Jones, KA; Mukherji, S; Cahill, ME; VanLeeuwen, JE; Woolfrey, KM; Rafalovich, I; Swanson, GT; Penzes, P
The Journal of biological chemistry
287
35964-74
2012
概要を表示する
The dendritic field of a neuron, which is determined by both dendritic architecture and synaptic strength, defines the synaptic input of a cell. Once established, a neuron's dendritic field is thought to remain relatively stable throughout a cell's lifetime. Perturbations in a dendritic structure or excitatory tone of a cell and thus its dendritic field are cellular alterations thought to be correlated with a number of psychiatric disorders. Although several proteins are known to regulate the development of dendritic arborization, much less is known about the mechanisms that maintain dendritic morphology and synaptic strength. In this study, we find that afadin, a component of N-cadherin·β-catenin·α-N-catenin adhesion complexes, is required for the maintenance of established dendritic arborization and synapse number. We further demonstrate that afadin directly interacts with AMPA receptors and that loss of this protein reduces the surface expression of GluA1- and GluA2-AMPA receptor subunits. Collectively, these data suggest that afadin is required for the maintenance of dendritic structure and excitatory tone. | Immunocytochemistry | | 22948147
 |
LRRK2 expression is enriched in the striosomal compartment of mouse striatum.
Mandemakers, W; Snellinx, A; O'Neill, MJ; de Strooper, B
Neurobiology of disease
48
582-93
2012
概要を表示する
In spite of a clear genetic link between Parkinson's disease (PD) and mutations in LRRK2, cellular localization and physiological function of LRRK2 remain debated. Here we demonstrate the immunohistochemical localization of LRRK2 in adult mouse and early postnatal mouse brain development. Antibody specificity is verified by absence of specific staining in LRRK2 knockout mouse brain. Although LRRK2 is expressed in various mouse brain regions (i.e. cortex, thalamus, hippocampus, cerebellum), strongest expression is detected in striatum, whereas LRRK2 protein expression in substantia nigra pars compacta in contrast is low. LRRK2 is highly expressed in striatal medium spiny neurons (MSN) and few cholinergic interneurons. LRRK2 expression is undetectable in other interneurons, oligodendrocytes or astrocytes of the striatum. Interestingly, LRRK2 expression is associated with striosome specific markers (i.e. MOR1, RASGRP1). Analysis of LRRK2 expression during early postnatal development and in LRRK2 knockout mice, demonstrates that LRRK2 is not required for generation or maintenance of the striosome compartment. Comparing LRRK2-WT, LRRK2-R1441G transgenic and non-transgenic mice, changes of LRRK2 expression in striosome/matrix compartments can be detected. The findings rule out a specific requirement of LRRK2 in striosome genesis but suggest a functional role for LRRK2 in striosomes. | Immunoblotting (Western) | | 22850484
 |
Experience-dependent regulation of NG2 progenitors in the developing barrel cortex.
Mangin, JM; Li, P; Scafidi, J; Gallo, V
Nature neuroscience
15
1192-4
2012
概要を表示する
We found that, during the formation of the mouse barrel cortex, NG2 cells received glutamatergic synapses from thalamocortical fibers and preferentially accumulated along septa separating the barrels. Sensory deprivation reduced thalamocortical inputs on NG2 cells and increased their proliferation, leading to a more uniform distribution in the deprived barrels. Thus, early sensory experience regulates thalamocortical innervation on NG2 cells, as well as their proliferation and distribution during development. | Immunofluorescence | | 22885848
 |
Social, Communication, and Cortical Structural Impairments in Epac2-Deficient Mice.
Srivastava, Deepak P, et al.
J. Neurosci., 32: 11864-11878 (2012)
2012
概要を表示する
Deficits in social and communication behaviors are common features of a number of neurodevelopmental disorders. However, the molecular and cellular substrates of these higher order brain functions are not well understood. Here we report that specific alterations in social and communication behaviors in mice occur as a result of loss of the EPAC2 gene, which encodes a protein kinase A-independent cAMP target. Epac2-deficient mice exhibited robust deficits in social interactions and ultrasonic vocalizations, but displayed normal olfaction, working and reference memory, motor abilities, anxiety, and repetitive behaviors. Epac2-deficient mice displayed abnormal columnar organization in the anterior cingulate cortex, a region implicated in social behavior in humans, but not in somatosensory cortex. In vivo two-photon imaging revealed reduced dendritic spine motility and density on cortical neurons in Epac2-deficient mice, indicating deficits at the synaptic level. Together, these findings provide novel insight into the molecular and cellular substrates of social and communication behavior. | | | 22915127
 |
Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling.
Neal, AP; Stansfield, KH; Worley, PF; Thompson, RE; Guilarte, TR
Toxicological sciences : an official journal of the Society of Toxicology
116
249-63
2010
概要を表示する
Lead (Pb(2+)) exposure is known to affect presynaptic neurotransmitter release in both in vivo and cell culture models. However, the precise mechanism by which Pb(2+) impairs neurotransmitter release remains unknown. In the current study, we show that Pb(2+) exposure during synaptogenesis in cultured hippocampal neurons produces the loss of synaptophysin (Syn) and synaptobrevin (Syb), two proteins involved in vesicular release. Pb(2+) exposure also increased the number of presynaptic contact sites. However, many of these putative presynaptic contact sites lack Soluble NSF attachment protein receptor complex proteins involved in vesicular exocytosis. Analysis of vesicular release using FM 1-43 dye confirmed that Pb(2+) exposure impaired vesicular release and reduced the number of fast-releasing sites. Because Pb(2+) is a potent N-methyl-D-aspartate receptor (NMDAR) antagonist, we tested the hypothesis that NMDAR inhibition may be producing the presynaptic effects. We show that NMDAR inhibition by aminophosphonovaleric acid mimics the presynaptic effects of Pb(2+) exposure. NMDAR activity has been linked to the signaling of the transsynaptic neurotrophin brain-derived neurotrophic factor (BDNF), and we observed that both the cellular expression of proBDNF and release of BDNF were decreased during the same period of Pb(2+) exposure. Furthermore, exogenous addition of BDNF rescued the presynaptic effects of Pb(2+). We suggest that the presynaptic deficits resulting from Pb(2+) exposure during synaptogenesis are mediated by disruption of NMDAR-dependent BDNF signaling. | Immunocytochemistry | | 20375082
 |