Nerve terminal degeneration is independent of muscle fiber genotype in SOD1 mice.
Carrasco, DI; Bichler, EK; Seburn, KL; Pinter, MJ
PloS one
5
e9802
2010
概要を表示する
Motor neuron degeneration in SOD1(G93A) transgenic mice begins at the nerve terminal. Here we examine whether this degeneration depends on expression of mutant SOD1 in muscle fibers.Hindlimb muscles were transplanted between wild-type and SOD1(G93A) transgenic mice and the innervation status of neuromuscular junctions (NMJs) was examined after 2 months. The results showed that muscles from SOD1(G93A) mice did not induce motor terminal degeneration in wildtype mice and that muscles from wildtype mice did not prevent degeneration in SOD1(G93A) transgenic mice. Control studies demonstrated that muscles transplanted from SOD1(G93A) mice continued to express mutant SOD1 protein. Experiments on wildtype mice established that the host supplied terminal Schwann cells (TSCs) at the NMJs of transplanted muscles.These results indicate that expression of the mutant protein in muscle is not needed to cause motor terminal degeneration in SOD1(G93A) transgenic mice and that a combination of motor terminals, motor axons and Schwann cells, all of which express mutant protein may be sufficient. | 20339550
 |
Adherent monomer-misfolded SOD1.
Watanabe, Y; Morita, E; Fukada, Y; Doi, K; Yasui, K; Kitayama, M; Nakano, T; Nakashima, K
PloS one
3
e3497
2008
概要を表示する
Multiple cellular functions are compromised in amyotrophic lateral sclerosis (ALS). In familial ALS (FALS) with Cu/Zn superoxide dismutase (SOD1) mutations, the mechanisms by which the mutation in SOD1 leads to such a wide range of abnormalities remains elusive.To investigate underlying cellular conditions caused by the SOD1 mutation, we explored mutant SOD1-interacting proteins in the spinal cord of symptomatic transgenic mice expressing a mutant SOD1, SOD1(Leu126delTT) with a FLAG sequence (DF mice). This gene product is structurally unable to form a functional homodimer. Tissues were obtained from both DF mice and disease-free mice expressing wild-type with FLAG SOD1 (WF mice). Both FLAG-tagged SOD1 and cross-linking proteins were enriched and subjected to a shotgun proteomic analysis. We identified 34 proteins (or protein subunits) in DF preparations, while in WF preparations, interactions were detected with only 4 proteins.These results indicate that disease-causing mutant SOD1 likely leads to inadequate protein-protein interactions. This could be an early and crucial process in the pathogenesis of FALS. 記事全文 | 18946506
 |
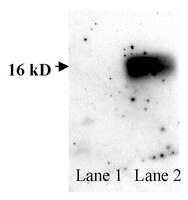
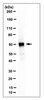
Development and characterization of human and mouse specific antibodies to CuZn-superoxide dismutase (SOD1).
Bartlett, S E, et al.
J. Neurosci. Methods, 98: 63-7 (2000)
2000
概要を表示する
Mutations in the copper/zinc superoxide dismutase (SOD1) gene are associated with 15-20% of the familial forms of motor neuron disease. Mice where a transgene has been incorporated that encodes for the human SOD1 mutation develop a form of motor neurone disease that closely resembles human forms of this disease. We have produced and characterized species-specific antibodies to epitopes in the SOD1 protein, amino acids 25-37, a region that distinguishes between the human and the mouse species of SOD1. The antisera generated were unable to immunoprecipitate the mouse or the human forms of SOD1 from tissue extracts unless the homodimeric complex of SOD1 was denatured. As SOD1 exists as a homodimeric complex in the cytoplasm of cells, this suggests that amino acids in position, 25-37 are close to the dimeric interface of SOD1. | 10837872
 |