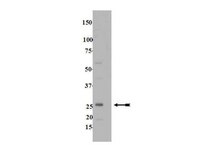
Rab13 mediates the continuous endocytic recycling of occludin to the cell surface.
Morimoto, Shinya, et al.
J. Biol. Chem., 280: 2220-8 (2005)
2005
概要を表示する
During epithelial morphogenesis, adherens junctions (AJs) and tight junctions (TJs) undergo dynamic reorganization, whereas epithelial polarity is transiently lost and reestablished. Although ARF6-mediated endocytic recycling of E-cadherin has been characterized and implicated in the rapid remodeling of AJs, the molecular basis for the dynamic rearrangement of TJs remains elusive. Occludin and claudins are integral membrane proteins comprising TJ strands and are thought to be responsible for establishing and maintaining epithelial polarity. Here we investigated the intracellular transport of occludin and claudins to and from the cell surface. Using cell surface biotinylation and immunofluorescence, we found that a pool of occludin was continuously endocytosed and recycled back to the cell surface in both fibroblastic baby hamster kidney cells and epithelial MTD-1A cells. Biochemical endocytosis and recycling assays revealed that a Rab13 dominant active mutant (Rab13 Q67L) inhibited the postendocytic recycling of occludin, but not that of transferrin receptor and polymeric immunoglobulin receptor in MTD-1A cells. Double immunolabelings showed that a fraction of endocytosed occludin was colocalized with Rab13 in MTD-1A cells. These results suggest that Rab13 specifically mediates the continuous endocytic recycling of occludin to the cell surface in both fibroblastic and epithelial cells. | 15528189
 |
Rab13 regulates PKA signaling during tight junction assembly.
Köhler, Katja, et al.
J. Cell Biol., 165: 175-80 (2004)
2004
概要を表示する
The GTPase Rab13 regulates the assembly of functional epithelial tight junctions (TJs) through a yet unknown mechanism. Here, we show that expression of the GTP-bound form of Rab13 inhibits PKA-dependent phosphorylation and TJ recruitment of the vasodilator-stimulated phosphoprotein, an actin remodelling protein. We demonstrate that Rab13GTP directly binds to PKA and inhibits its activity. Interestingly, activation of PKA abrogates the inhibitory effect of Rab13 on the recruitment of vasodilator-stimulated phosphoprotein, ZO-1, and claudin1 to cell-cell junctions. Rab13 is, therefore, the first GTPase that controls PKA activity and provides an unexpected link between PKA signaling and the dynamics of TJ assembly. | 15096524
 |