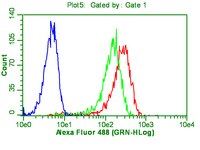
Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment.
Grymula, K; Tarnowski, M; Piotrowska, K; Suszynska, M; Mierzejewska, K; Borkowska, S; Fiedorowicz, K; Kucia, M; Ratajczak, MZ
Journal of cellular and molecular medicine
18
1797-806
2014
概要を表示する
The concept that bone marrow (BM)-derived cells may participate in neural regeneration remains controversial, and the identity of the specific cell type(s) involved remains unknown. We recently reported that the adult murine BM contains a highly mobile population of Sca-1(+) Lin(-) CD45(-) cells known as very small embryonic/epiblast-like stem cells (VSELs) that express several markers of pluripotency such as Oct-4. In the BM microenvironment, these cells are kept quiescent because of epigenetic modification of certain paternally imprinted genes. However, as reported, these cells can be mobilized in mice in an experimental model of stroke and express several genes involved in neurogenesis while circulating in peripheral blood (PB). In the current work, we employed a model of toxic brain damage, which is induced by administration of kainic acid, to see not only whether VSELs can be mobilized into PB in response to this neurotoxin, but, more importantly, whether they proliferate and expand in BM tissue. We report here for the first time that brain damage leads to activation and expansion of the BM pool of quiescent VSELs, which precedes their subsequent egress into PB. Harnessing these cells in neural tissue regeneration is currently one of the challenges in regenerative medicine. | | 24895014
 |
Improving outcomes of acute kidney injury using mouse renal progenitor cells alone or in combination with erythropoietin or suramin.
Han, X; Zhao, L; Lu, G; Ge, J; Zhao, Y; Zu, S; Yuan, M; Liu, Y; Kong, F; Xiao, Z; Zhao, S
Stem cell research & therapy
4
74
2013
概要を表示する
So far, no effective therapy is available for acute kidney injury (AKI), a common and serious complication with high morbidity and mortality. Interest has recently been focused on the potential therapeutic effect of mouse adult renal progenitor cells (MRPC), erythropoietin (EPO) and suramin in the recovery of ischemia-induced AKI. The aim of the present study is to compare MRPC with MRPC/EPO or MRPC/suramin concomitantly in the treatment of a mouse model of ischemia/reperfusion (I/R) AKI.MRPC were isolated from adult C57BL/6-gfp mice. Male C57BL/6 mice (eight-weeks old, n = 72) were used for the I/R AKI model. Serum creatinine (Cr), blood urea nitrogen (BUN) and renal histology were detected in MRPC-, MRPC/EPO-, MRPC/suramin- and PBS-treated I/R AKI mice. E-cadherin, CD34 and GFP protein expression was assessed by immunohistochemical assay.MRPC exhibited characteristics consistent with renal stem cells. The features of MRPC were manifested by Pax-2, Oct-4, vimentin, α-smooth muscle actin positive, and E-cadherin negative, distinguished from mesenchymal stem cells (MSC) by expression of CD34 and Sca-1. The plasticity of MRPC was shown by the ability to differentiate into osteoblasts and lipocytes in vitro. Injection of MRPC, especially MRPC/EPO and MRPC/suramin in I/R AKI mice attenuated renal damage with a decrease of the necrotic injury, peak plasma Cr and BUN. Furthermore, seven days after the injury, MRPC/EPO or MRPC/suramin formed more CD34(+) and E-cadherin+ cells than MRPC alone.These results suggest that MRPC, in particular MRPC/EPO or MRPC/suramin, promote renal repair after injury and may be a promising therapeutic strategy. | | 23777889
 |
Molecular signatures to define spermatogenic cells in common marmoset (Callithrix jacchus).
Lin, ZY; Imamura, M; Sano, C; Nakajima, R; Suzuki, T; Yamadera, R; Takehara, Y; Okano, HJ; Sasaki, E; Okano, H
Reproduction (Cambridge, England)
143
597-609
2012
概要を表示する
Germ cell development is a fundamental process required to produce offspring. The developmental program of spermatogenesis has been assumed to be similar among mammals. However, recent studies have revealed differences in the molecular properties of primate germ cells compared with the well-characterized mouse germ cells. This may prevent simple application of rodent insights into higher primates. Therefore, thorough investigation of primate germ cells is necessary, as this may lead to the development of more appropriate animal models. The aim of this study is to define molecular signatures of spermatogenic cells in the common marmoset, Callithrix jacchus. Interestingly, NANOG, PRDM1, DPPA3 (STELLA), IFITM3, and ZP1 transcripts, but no POU5F1 (OCT4), were detected in adult marmoset testis. Conversely, mouse testis expressed Pou5f1 but not Nanog, Prdm1, Dppa3, Ifitm3, and Zp1. Other previously described mouse germ cell markers were conserved in marmoset and mouse testes. Intriguingly, marmoset spermatogenic cells underwent dynamic protein expression in a developmental stage-specific manner; DDX4 (VASA) protein was present in gonocytes, diminished in spermatogonial cells, and reexpressed in spermatocytes. To investigate epigenetic differences between adult marmoset and mice, DNA methylation analyses identified unique epigenetic profiles to marmoset and mice. Marmoset NANOG and POU5F1 promoters in spermatogenic cells exhibited a methylation status opposite to that in mice, while the DDX4 and LEFTY1 loci, as well as imprinted genes, displayed an evolutionarily conserved methylation pattern. Marmosets have great advantages as models for human reproductive biology and are also valuable as experimental nonhuman primates; thus, the current study provides an important platform for primate reproductive biology, including possible applications to humans. | | 22323619
 |
Global and gene-specific histone modification profiles of mouse multipotent adult germline stem cells.
Khromov, T; Pantakani, DV; Nolte, J; Wolf, M; Dressel, R; Engel, W; Zechner, U
Molecular human reproduction
17
166-74
2011
概要を表示する
We previously reported the generation of multipotent adult germline stem cells (maGSCs) from spermatogonial stem cells (SSCs) isolated from adult mouse testis. In a later study, we substantiated the pluripotency of maGSCs by demonstrating their close similarity to pluripotent male embryonic stem cells (ESCs) at the epigenetic level of global and gene-specific DNA methylation. Here, we extended the comparative epigenetic analysis of maGSCs and male ESCs by investigating the second main epigenetic modification in mammals, i.e. global and gene-specific modifications of histones (H3K4 trimethylation, H3K9 acetylation, H3K9 trimethylation and H3K27 trimethylation). Using immunofluorescence staining, flow cytometry and western blot analysis, we show that maGSCs are very similar to male ESCs with regard to global levels and nuclear distribution patterns of these modifications. Chromatin immunoprecipitation real-time PCR analysis of these modifications at the gene-specific level further revealed modification patterns of the pluripotency marker genes Oct4, Sox2 and Nanog in maGSCs that are nearly identical to those of male ESCs. These genes were enriched for activating histone modifications including H3K4me3 and H3K9ac and depleted of repressive histone modifications including H3K27me3 and H3K9me3. In addition, Hoxa11, a key regulator of early embryonic development showed the ESC-typical bivalent chromatin conformation with enrichment of both the activating H3K4me3 and the repressive H3K27me3 modification also in maGSCs. Collectively, our results demonstrate that maGSCs also closely resemble ESCs with regard to their chromatin state and further evidence their pluripotent nature. | Immunofluorescence | 20935159
 |
Gene targeting and subsequent site-specific transgenesis at the ?-actin (ACTB) locus in common marmoset embryonic stem cells.
Seiji Shiozawa,Kenji Kawai,Yohei Okada,Ikuo Tomioka,Takuji Maeda,Akifumi Kanda,Haruka Shinohara,Hiroshi Suemizu,Hirotaka James Okano,Yusuke Sotomaru,Erika Sasaki,Hideyuki Okano
Stem cells and development
20
2011
概要を表示する
Nonhuman primate embryonic stem (ES) cells have vast promise for preclinical studies. Genetic modification in nonhuman primate ES cells is an essential technique for maximizing the potential of these cells. The common marmoset (Callithrix jacchus), a nonhuman primate, is expected to be a useful transgenic model for preclinical studies. However, genetic modification in common marmoset ES (cmES) cells has not yet been adequately developed. To establish efficient and stable genetic modifications in cmES cells, we inserted the enhanced green fluorescent protein (EGFP) gene with heterotypic lox sites into the ?-actin (ACTB) locus of the cmES cells using gene targeting. The resulting knock-in ES cells expressed EGFP ubiquitously under the control of the endogenous ACTB promoter. Using inserted heterotypic lox sites, we demonstrated Cre recombinase-mediated cassette exchange (RMCE) and successfully established a monomeric red fluorescent protein (mRFP) knock-in cmES cell line. Further, a herpes simplex virus-thymidine kinase (HSV-tk) knock-in cmES cell line was established using RMCE. The growth of tumor cells originating from the cell line was significantly suppressed by the administration of ganciclovir. Therefore, the HSV-tk/ganciclovir system is promising as a safeguard for stem cell therapy. The stable and ubiquitous expression of EGFP before RMCE enables cell fate to be tracked when the cells are transplanted into an animal. Moreover, the creation of a transgene acceptor locus for site-specific transgenesis will be a powerful tool, similar to the ROSA26 locus in mice. | | 21126169
 |