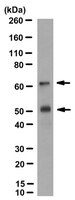
Activation of OASIS family, ER stress transducers, is dependent on its stabilization.
Kondo, S; Hino, SI; Saito, A; Kanemoto, S; Kawasaki, N; Asada, R; Izumi, S; Iwamoto, H; Oki, M; Miyagi, H; Kaneko, M; Nomura, Y; Urano, F; Imaizumi, K
Cell death and differentiation
19
1939-49
2012
概要を表示する
Endoplasmic reticulum (ER) stress transducers transduce signals from the ER to the cytoplasm and nucleus when unfolded proteins accumulate in the ER. BBF2 human homolog on chromosome 7 (BBF2H7) and old astrocyte specifically induced substance (OASIS), ER-resident transmembrane proteins, have recently been identified as novel ER stress transducers that have roles in chondrogenesis and osteogenesis, respectively. However, the molecular mechanisms that regulate the activation of BBF2H7 and OASIS under ER stress conditions remain unresolved. Here, we showed that BBF2H7 and OASIS are notably unstable proteins that are easily degraded via the ubiquitin-proteasome pathway under normal conditions. ER stress conditions enhanced the stability of BBF2H7 and OASIS, and promoted transcription of their target genes. HMG-CoA reductase degradation 1 (HRD1), an ER-resident E3 ubiquitin ligase, ubiquitinated BBF2H7 and OASIS under normal conditions, whereas ER stress conditions dissociated the interaction between HRD1 and BBF2H7 or OASIS. The stabilization of OASIS in Hrd1(-/-) cells enhanced the expression of collagen fibers during osteoblast differentiation, whereas a knockdown of OASIS in Hrd1(-/-) cells suppressed the production of collagen fibers. These findings suggest that ER stress stabilizes OASIS family members and this is a novel molecular mechanism for the activation of ER stress transducers. | 22705851
 |
Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation.
Murakami, T; Saito, A; Hino, S; Kondo, S; Kanemoto, S; Chihara, K; Sekiya, H; Tsumagari, K; Ochiai, K; Yoshinaga, K; Saitoh, M; Nishimura, R; Yoneda, T; Kou, I; Furuichi, T; Ikegawa, S; Ikawa, M; Okabe, M; Wanaka, A; Imaizumi, K
Nature cell biology
11
1205-11
2009
概要を表示する
Eukaryotic cells have signalling pathways from the endoplasmic reticulum (ER) to cytosol and nuclei, to avoid excess accumulation of unfolded proteins in the ER. We previously identified a new type of ER stress transducer, OASIS, a bZIP (basic leucine zipper) transcription factor, which is a member of the CREB/ATF family and has a transmembrane domain. OASIS is processed by regulated intramembrane proteolysis (RIP) in response to ER stress, and is highly expressed in osteoblasts. OASIS(-/-) mice exhibited severe osteopenia, involving a decrease in type I collagen in the bone matrix and a decline in the activity of osteoblasts, which showed abnormally expanded rough ER, containing of a large amount of bone matrix proteins. Here we identify the gene for type 1 collagen, Col1a1, as a target of OASIS, and demonstrate that OASIS activates the transcription of Col1a1 through an unfolded protein response element (UPRE)-like sequence in the osteoblast-specific Col1a1 promoter region. Moreover, expression of OASIS in osteoblasts is induced by BMP2 (bone morphogenetic protein 2), the signalling of which is required for bone formation. Additionally, RIP of OASIS is accelerated by BMP2 signalling, which causes mild ER stress. Our studies show that OASIS is critical for bone formation through the transcription of Col1a1 and the secretion of bone matrix proteins, and they reveal a new mechanism by which ER stress-induced signalling mediates bone formation. | 19767743
 |