Slit diaphragm protein Neph1 and its signaling: a novel therapeutic target for protection of podocytes against glomerular injury.
Arif, E; Rathore, YS; Kumari, B; Ashish, F; Wong, HN; Holzman, LB; Nihalani, D
The Journal of biological chemistry
289
9502-18
2014
概要を表示する
Podocytes are specialized epithelial cells that are critical components of the glomerular filtration barrier, and their dysfunction leads to proteinuria and renal failure. Therefore, preserving podocyte function is therapeutically significant. In this study, we identified Neph1 signaling as a therapeutic target that upon inhibition prevented podocyte damage from a glomerular injury-inducing agent puromycin aminonucleoside (PAN). To specifically inhibit Neph1 signaling, we used a protein transduction approach, where the cytoplasmic domain of Neph1 (Neph1CD) tagged with a protein transduction domain trans-activator of transcription was transduced in cultured podocytes prior to treatment with PAN. The PAN-induced Neph1 phosphorylation was significantly reduced in Neph1CD-transduced cells; in addition, these cells were resistant to PAN-induced cytoskeletal damage. The biochemical analysis using subfractionation studies showed that unlike control cells Neph1 was retained in the lipid raft fractions in the transduced cells following treatment with PAN, indicating that transduction of Neph1CD in podocytes prevented PAN-induced mislocalization of Neph1. In accordance, the immunofluorescence analysis further suggested that Neph1CD-transduced cells had increased ability to retain endogenous Neph1 at the membrane in response to PAN-induced injury. Similar results were obtained when angiotensin was used as an injury-inducing agent. Consistent with these observations, maintaining high levels of Neph1 at the membrane using a podocyte cell line overexpressing chimeric Neph1 increased the ability of podocytes to resist PAN-induced injury and PAN-induced albumin leakage. Using a zebrafish in vivo PAN and adriamycin injury models, we further demonstrated the ability of transduced Neph1CD to preserve glomerular function. Collectively, these results support the conclusion that inhibiting Neph1 signaling is therapeutically significant in preventing podocyte damage from glomerular injury. | 24554715
 |
Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane.
Arif, E; Wagner, MC; Johnstone, DB; Wong, HN; George, B; Pruthi, PA; Lazzara, MJ; Nihalani, D
Molecular and cellular biology
31
2134-50
2011
概要を表示する
The podocyte proteins Neph1 and nephrin organize a signaling complex at the podocyte cell membrane that forms the structural framework for a functional glomerular filtration barrier. Mechanisms regulating the movement of these proteins to and from the membrane are currently unknown. This study identifies a novel interaction between Neph1 and the motor protein Myo1c, where Myo1c plays an active role in targeting Neph1 to the podocyte cell membrane. Using in vivo and in vitro experiments, we provide data supporting a direct interaction between Neph1 and Myo1c which is dynamic and actin dependent. Unlike wild-type Myo1c, the membrane localization of Neph1 was significantly reduced in podocytes expressing dominant negative Myo1c. In addition, Neph1 failed to localize at the podocyte cell membrane and cell junctions in Myo1c-depleted podocytes. We further demonstrate that similarly to Neph1, Myo1c also binds nephrin and reduces its localization at the podocyte cell membrane. A functional analysis of Myo1c knockdown cells showed defects in cell migration, as determined by a wound assay. In addition, the ability to form tight junctions was impaired in Myo1c knockdown cells, as determined by transepithelial electric resistance (TER) and bovine serum albumin (BSA) permeability assays. These results identify a novel Myo1c-dependent molecular mechanism that mediates the dynamic organization of Neph1 and nephrin at the slit diaphragm and is critical for podocyte function. | 21402783
 |
Ischemic injury to kidney induces glomerular podocyte effacement and dissociation of slit diaphragm proteins Neph1 and ZO-1.
Wagner, MC; Rhodes, G; Wang, E; Pruthi, V; Arif, E; Saleem, MA; Wean, SE; Garg, P; Verma, R; Holzman, LB; Gattone, V; Molitoris, BA; Nihalani, D
The Journal of biological chemistry
283
35579-89
2008
概要を表示する
Glomerular injury is often characterized by the effacement of podocytes, loss of slit diaphragms, and proteinuria. Renal ischemia or the loss of blood flow to the kidneys has been widely associated with tubular and endothelial injury but rarely has been shown to induce podocyte damage and disruption of the slit diaphragm. In this study, we have used an in vivo rat ischemic model to demonstrate that renal ischemia induces podocyte effacement with loss of slit diaphragm and proteinuria. Biochemical analysis of the ischemic glomerulus shows that ischemia induces rapid loss of interaction between slit diaphragm junctional proteins Neph1 and ZO-1. To further understand the effect of ischemia on molecular interactions between slit diaphragm proteins, a cell culture model was employed to study the binding between Neph1 and ZO-1. Under physiologic conditions, Neph1 co-localized with ZO-1 at cell-cell contacts in cultured human podocytes. Induction of injury by ATP depletion resulted in rapid loss of Neph1 and ZO-1 binding and redistribution of Neph1 and ZO-1 proteins from cell membrane to the cytoplasm. Recovery resulted in increased Neph1 tyrosine phosphorylation, restoring Neph1 and ZO-1 binding and their localization at the cell membrane. We further demonstrate that tyrosine phosphorylation of Neph1 mediated by Fyn results in significantly increased Neph1 and ZO-1 binding, suggesting a critical role for Neph1 tyrosine phosphorylation in reorganizing the Neph1-ZO-1 complex. This study documents that renal ischemia induces dynamic changes in the molecular interactions between slit diaphragm proteins, leading to podocyte damage and proteinuria. | 18922801
 |
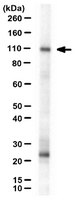
Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers.
Barletta, GM; Kovari, IA; Verma, RK; Kerjaschki, D; Holzman, LB
The Journal of biological chemistry
278
19266-71
2003
概要を表示する
Glomerular visceral epithelial cells (podocytes) appear to play a central role in maintaining the selective filtration barrier of the renal glomerulus. While the immunoglobulin superfamily member Nephrin was proposed to act as a cell adhesion molecule at the podocyte intercellular junction necessary for maintaining glomerular perm selectivity, the Nephrin ligand has not been identified. The existence of a new subfamily of Nephrin-like molecules including Neph1 was recently described. Genetic deletion of Nephrin or Neph1 resulted in similar phenotypes of podocyte foot process effacement and proteinuria. The subcellular localization of Neph1 and the possibility that Nephrin and Neph1 interact was investigated. Polyclonal antiserum for Neph1 was raised and characterized. Neph1 migrated as a 90-kDa protein on SDS-PAGE under reducing conditions. Neph1 was identified in a glomerular and podocyte-specific distribution in adult rat kidney. Like Nephrin and Podocin, Neph1 was enriched in Triton X-100 detergent-resistant membrane fractions. Consistent with this observation, immunogold electron microscopy demonstrated that Neph1 localized exclusively to lateral margins of podocyte foot processes at the insertion of the slit diaphragm. Neph1 and Nephrin participate in a direct cis-interaction involving their cytoplasmic domains. In addition, interactions between the extracellular domain of Nephrin and itself and between the extracellular domain of Nephrin and that of Neph1 were detected. Neph1 did not interact via a homophilic interaction. These observations suggest that Nephrin and Neph1 form a hetero-oligomeric receptor complex in the plane of the membrane that might interact across the foot process intercellular junction through interactions between Nephrin with itself and Neph1. | 12646566
 |
Nephrin localizes to the slit pore of the glomerular epithelial cell.
Holzman, LB; St John, PL; Kovari, IA; Verma, R; Holthofer, H; Abrahamson, DR
Kidney international
56
1481-91
1999
概要を表示する
Recognition that mutation of the protein nephrin, encoded by the NPHS1 gene, singly results in the cellular alterations that result in foot process effacement, and nephrotic range proteinuria emphasizes the pivotal role that this protein plays in regulating glomerular filter integrity. This article reports the development of reagents necessary to study the biology of nephrin in mouse, and describes the initial characterization of the nephrin protein.A cDNA including the full-length mouse nephrin open reading frame was cloned and sequenced. Immuno-affinity purified polyclonal antiserum directed against the cytoplasmic domain of mouse nephrin was developed.Nephrin identified in mouse glomerular extract was found to be a glycoprotein with an apparent molecular mass of 185 kDa. As detected by indirect immunofluorescence microscopy and immunogold electron microscopy, nephrin was located only in visceral glomerular epithelial cells, where it was targeted to intercellular junctions of mature podocyte foot processes. In developing glomeruli of newborn mouse, antinephrin immunolocalized to the earliest slit pore regions between differentiating podocytes, sites where slit diaphragms first become visible.As a putative cell adhesion molecule of the immunoglobulin superfamily, nephrin likely participates in cell-cell interactions between podocyte foot processes and may represent a component of the slit diaphragm. | 10504499
 |