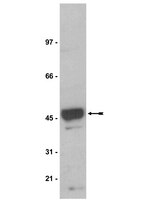
Apical sorting of lysoGPI-anchored proteins occurs independent of association with detergent-resistant membranes but dependent on their N-glycosylation.
Castillon, GA; Michon, L; Watanabe, R
Molecular biology of the cell
24
2021-33
2013
概要を表示する
Most glycosylphosphatidylinositol-anchored proteins (GPI-APs) are located at the apical surface of epithelial cells. The apical delivery of GPI-APs is believed to result from their association with lipid rafts. We find that overexpression of C-terminally tagged PGAP3 caused predominant production of lysoGPI-APs, an intermediate precursor in the GPI lipid remodeling process in Madin-Darby canine kidney cells. In these cells, produced lysoGPI-APs are not incorporated into detergent-resistant membranes (DRMs) but still are delivered apically, suggesting that GPI-AP association with DRMs is not necessary for apical targeting. In contrast, apical transport of both fully remodeled and lyso forms of GPI-APs is dependent on N-glycosylation, confirming a general role of N-glycans in apical protein transport. We also find that depletion of cholesterol causes apical-to-basolateral retargeting not only of fully remodeled GPI-APs, but also of lysoGPI-APs, as well as endogenous soluble and transmembrane proteins that would normally be targeted to the apical membrane. These findings confirm the essential role for cholesterol in the apical protein targeting and further demonstrate that the mechanism of cholesterol-dependent apical sorting is not related to DRM association of GPI-APs. | | 23615438
 |
Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death.
Lee J Martin,Zhiping Liu,Kevin Chen,Ann C Price,Yan Pan,Jason A Swaby,W Christopher Golden
The Journal of comparative neurology
500
2007
概要を表示する
The mechanisms of human mutant superoxide dismutase-1 (mSOD1) toxicity to motor neurons (MNs) are unresolved. We show that MNs in G93A-mSOD1 transgenic mice undergo slow degeneration lacking similarity to apoptosis structurally and biochemically. It is characterized by somal and mitochondrial swelling and formation of DNA single-strand breaks prior to double-strand breaks occurring in nuclear and mitochondrial DNA. p53 and p73 are activated in degenerating MNs, but without nuclear import. The MN death is independent of activation of caspases-1, -3, and -8 or apoptosis-inducing factor within MNs, with a blockade of apoptosis possibly mediated by Aven up-regulation. MN swelling is associated with compromised Na,K-ATPase activity and aggregation. mSOD1 mouse MNs accumulate mitochondria from the axon terminals and generate higher levels of superoxide, nitric oxide, and peroxynitrite than MNs in control mice. Nitrated and aggregated cytochrome c oxidase subunit-I and alpha-synuclein as well as nitrated SOD2 accumulate in mSOD1 mouse spinal cord. Mitochondria in mSOD1 mouse MNs accumulate NADPH diaphorase and inducible nitric oxide synthase (iNOS)-like immunoreactivity, and iNOS gene deletion extends significantly the life span of G93A-mSOD1 mice. Prior to MN loss, spinal interneurons degenerate. These results identify novel mechanisms for mitochondriopathy and MN degeneration in amyotrophic lateral sclerosis (ALS) mice involving blockade of apoptosis, accumulation of MN mitochondria with enhanced toxic potential from distal terminals, NOS localization in MN mitochondria and peroxynitrite damage, and early degeneration of alpha-synuclein(+) interneurons. The data support roles for oxidative stress, protein nitration and aggregation, and excitotoxicity as participants in the process of MN degeneration caused by mSOD1. | | 17099894
 |
Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells.
Jianxun Lei, Sogol Nowbar, Cary N Mariash, David H Ingbar
American journal of physiology. Lung cellular and molecular physiology
285
L762-72
2003
概要を表示する
Na-K-ATPase protein is critical for maintaining cellular ion gradients and volume and for transepithelial ion transport in kidney and lung. Thyroid hormone, 3,3',5-triiodo-l-thyronine (T3), given for 2 days to adult rats, increases alveolar fluid resorption by 65%, but the mechanism is undefined. We tested the hypothesis that T3 stimulates Na-K-ATPase in adult rat alveolar epithelial cells (AEC), including primary rat alveolar type II (ATII) cells, and determined mechanisms of the T3 effect on the Na-KATPase enzyme using two adult rat AEC cell lines (MP48 and RLE-6TN). T3 at 10-8 and 10-5 M increased significantly hydrolytic activity of Na-K-ATPase in primary ATII cells and both AEC cell lines. The increased activity was dose dependent in the cell lines (10-9-10-4 M) and was detected within 30 min and peaked at 6 h. Maximal increases in Na-K-ATPase activity were twofold in MP48 and RLE-6TN cells at pharmacological T3 of 10-5 and 10-4 M, respectively, but increases were statistically significant at physiological T3 as low as 10-9 M. This effect was T3 specific, because reverse T3 (3,3',5'-triiodo-l-thyronine) at 10-9-10-4 M had no effect. The T3-induced increase in Na-K-ATPase hydrolytic activity was not blocked by actinomycin D. No significant change in mRNA and total cell protein levels of Na-K-ATPase were detected with 10-9-10-5 M T3 at 6 h. However, T3 increased cell surface expression of Na-K-ATPase alpha1- or beta1-subunit proteins by 1.7- and 2-fold, respectively, and increases in Na-K-ATPase activity and cell surface expression were abolished by brefeldin A. These data indicate that T3 specifically stimulates Na-K-ATPase activity in adult rat AEC. The upregulation involves translocation of Na-K-ATPase to plasma membrane, not increased gene transcription. These results suggest a novel nontranscriptional mechanism for regulation of Na-K-ATPase by thyroid hormone. | | 12740220
 |
Regional distribution of Na,K-ATPase activity in porcine lens epithelium
Tamiya, S., et al
Invest Ophthalmol Vis Sci, 44:4395-9 (2003)
2003
| Immunoblotting (Western) | 14507885
 |
Analysis of subunit assembly of the Na-K-ATPase.
Fambrough, D M, et al.
Am. J. Physiol., 266: C579-89 (1994)
1994
概要を表示する
The Na-K-ATPase, or sodium pump, is comprised of two subunits, alpha and beta. Each subunit spans the lipid bilayer of the cell membrane. This review summarizes our efforts to determine how the two subunits interact to form the functional ion transporter. Our major approach has been to observe the potential for subunit assembly when one or both subunits are truncated or present as chimeras that retain only a limited region of the Na-K-ATPase. DNAs encoding these altered subunit forms of the avian Na-K-ATPase are expressed in mammalian cells. Monoclonal antibodies specific for the avian beta-subunit are then used to purify newly synthesized avian beta-subunits, and the presence of accompanying alpha-subunits indicates that subunit assembly has occurred. The ectodomain of the beta-subunit (approximately residues 62-304) is sufficient for assembly with the alpha-subunit, and a COOH-terminal truncation of the beta-subunit that lacks aminoacyl residues beyond 162 will assemble inefficiently. A maximum of 26 aminoacyl residues of the alpha-subunit are necessary for robust assembly with the beta-subunit, when this sequence replaces the COOH-terminal half of the loop between membrane spans 7 and 8 in the SERCA1 Ca-ATPase. This region of the Ca-ATPase faces the lumen of the endoplasmic reticulum. These findings encourage study of other related questions, including whether there is preferential assembly of certain subunit isoforms and how various P-type ATPases are targeted to their appropriate subcellular compartments. | | 8166221
 |
The alpha and beta subunits of the Na,K-ATPase can assemble at the plasma membrane into functional enzyme.
A W DeTomaso, G Blanco, R W Mercer
The Journal of cell biology
127
55-69
1994
概要を表示する
Synthesis and assembly of most oligomeric plasma membrane proteins occurs in the ER. However, the role the ER plays in oligomerization is unknown. We have previously demonstrated that unassociated alpha and beta subunits of the Na,K-ATPase are targeted to the plasma membrane when individually expressed in baculovirus-infected Sf-9 cells. This unique property allows us to determine if assembly of these two polypeptides is restricted to the ER, or if it can also occur at the plasma membrane. To investigate the assembly of the Na,K-ATPase we have taken advantage of the ability of baculovirus-infected cells to fuse. Lowering the extracellular pH of the infected cells triggers an endogenously expressed viral protein to initiate plasma membrane fusion. When individual Sf-9 cells expressing either the Na,K-ATPase alpha or beta subunits are plated together and subjected to a mild acidic shock, they form large syncytia. In the newly continuous plasma membrane the separate alpha and beta polypeptides associate and assemble into functional Na,K-ATPase molecules. However, a hybrid ATPase molecule consisting of a Na,K-ATPase alpha subunit and a H,K-ATPase beta subunit, which efficiently assembles in the ER of coinfected cells, does not assemble at the plasma membrane of fused cells. When cells expressing the Na,K-ATPase alpha subunit are fused to cells coexpressing the Na,K-ATPase beta subunit and the H,K-ATPase beta subunit, the Na,K-ATPase alpha subunit selectively assembles with the Na,K-ATPase beta subunit. However, when cells are coinfected and expressing all three polypeptides, the Na,K-ATPase alpha subunit assembles with both beta subunits in the ER, in what appears to be a random fashion. These experiments demonstrate that assembly between some polypeptides is restricted to the ER, and suggests that the ability of the Na,K-ATPase alpha and beta subunits to leave the ER and assemble at the plasma membrane may represent a novel mechanism of regulation of activity. 記事全文 | | 7929571
 |
Expression and molecular regulation of Na(+)-K(+)-ATPase after renal ischemia.
Van Why, S K, et al.
Am. J. Physiol., 267: F75-85 (1994)
1994
概要を表示する
Renal ischemia causes redistribution of Na(+)-K(+)-adenosinetriphosphatase (Na(+)-K(+)-ATPase) to the apical membrane of proximal tubules. We determined the time course of regeneration of Na(+)-K(+)-ATPase polarity and sought evidence of increased enzyme production during recovery as a means to restore polarity. Anesthetized rats underwent 45 min renal ischemia and reflow of 15 min, 2 h, 6 h, and 24 h. Immunofluorescent and electron microscopy showed loss of strict basolateral localization of Na(+)-K(+)-ATPase at 15 min reflow with repolarization by 24 h in sublethally injured cells. Both alpha 1- and beta-subunits were only in microsomal fractions at all reflow intervals. Immunodetectable levels of both subunits declined to 60-70% of control by 24 h reflow. Levels of mRNA for each subunit declined in parallel through 24 h to 55% of control. Overall transcription was profoundly depressed through 6 h but had recovered to near control by 24 h. Specific transcription of alpha 1- and beta-subunit mRNA was markedly decreased after ischemia and only partially recovered by 24 h. These results suggest that recycling of misplaced units rather than new Na(+)-K(+)-ATPase production is the means by which renal epithelia initially repolarize after ischemic injury. | | 8048568
 |
An ion-transporting ATPase encodes multiple apical localization signals.
Gottardi, C J and Caplan, M J
J. Cell Biol., 121: 283-93 (1993)
1993
概要を表示する
Epithelial cells accumulate distinct populations of membrane proteins at their two plasmalemmal domains. We have examined the molecular signals which specify the differential subcellular distributions of two closely related ion pumps. The Na,K-ATPase is normally restricted to the basolateral membranes of numerous epithelial cell types, whereas the H,K-ATPase is a component of the apical surfaces of the parietal cells of the gastric epithelium. We have expressed full length and chimeric H,K-ATPase/Na,K-ATPase cDNAs in polarized renal proximal tubular epithelial cells (LLC-PK1). We find that both the alpha and beta subunits of the H,K-ATPase encode independent signals that specify apical localization. Furthermore, the H,K-ATPase beta-subunit possesses a sequence which mediates its participation in the endocytic pathway. The interrelationship between epithelial sorting and endocytosis signals suggested by these studies supports the redefinition of apical and basolateral as functional, rather than simply topographic domains. | | 8385670
 |
Delivery of Na+,K(+)-ATPase in polarized epithelial cells.
Gottardi, C J and Caplan, M J
Science, 260: 552-4; author reply 554-6 (1993)
1993
| | 8386395
 |
Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly.
Gottardi, C J and Caplan, M J
J. Biol. Chem., 268: 14342-7 (1993)
1993
概要を表示する
The ion-transporting H,K-ATPase and Na,K-ATPase enzymes are each composed of an alpha and a beta subunit. It is known that assembly of the alpha and beta subunits of the Na,K-ATPase is necessary for the cell-surface delivery of the active enzyme. We have examined the molecular domains involved in the assembly of the H,K-ATPase and Na,K-ATPase alpha and beta subunits by expressing individual subunits and subunit chimeras in transiently transfected COS-1 cells. Our results demonstrate that the H,K-ATPase alpha subunit requires its beta subunit for efficient cell-surface expression, as determined by indirect immunofluorescence. The H,K-ATPase beta protein appears to be able to get to the cell surface unaccompanied by any alpha subunit and appears to localize as well to a population of intracellular vesicles. We find that a transfected chimera encoding the NH2-terminal half of the H,K-ATPase alpha subunit and the COOH-terminal half of the Na,K-ATPase alpha subunit appears to assemble with the endogenous Na,K-ATPase beta subunit and to reach the plasmalemma. Transfection of the complementary alpha chimera requires coexpression with the H,K-ATPase beta subunit in order to attain surface delivery. Thus, it is the COOH-terminal half of the alpha subunit that specifies assembly with a particular beta subunit. | | 8390991
 |