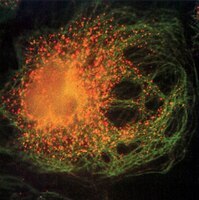
Oatp1a1 requires PDZK1 to traffic to the plasma membrane by selective recruitment of microtubule-based motor proteins.
Wang, WJ; Murray, JW; Wolkoff, AW
Drug metabolism and disposition: the biological fate of chemicals
42
62-9
2014
概要を表示する
Previous studies identified a family of organic anion transport proteins (OATPs), many of which have C-terminal PDZ binding consensus sequences. In particular, the C-terminal four amino acids of Oatp1a1, a transporter on rat and mouse hepatocytes, comprise a consensus binding site for PDZK1. In PDZK1 knockout mice and in transfected cells where PDZK1 expression was knocked down, Oatp1a1 accumulates in intracellular vesicles. The present study tests the hypothesis that Oatp1a1 traffics to and from the cell surface in vesicles along microtubules, and that PDZK1 guides recruitment of specific motors to these vesicles. Oatp1a1-containing vesicles were prepared from wild-type and PDZK1 knockout mice. As seen by immunofluorescence, kinesin-1, a microtubule plus-end directed motor, was largely associated with vesicles from wild-type mouse liver, whereas dynein, a minus-end directed motor, was largely associated with vesicles from PDZK1 knockout mouse liver. Quantification of motility on directionally marked microtubules following addition of 50 µM ATP showed that wild-type vesicles moved equally toward the plus and minus ends whereas PDZK1 knockout vesicles moved predominantly toward the minus end, consistent with net movement toward the cell interior. These studies provide a novel mechanism by which PDZK1 regulates intracellular trafficking of Oatp1a1 by recruiting specific motors to Oatp1a1-containing vesicles. In the absence of PDZK1, Oatp1a1-containing vesicles cannot recruit kinesin-1 and associate with dynein as a predominant minus-end directed motor. Whether this is a result of direct interaction of the Oatp1a1 cytoplasmic domain with dynein or with a dynein-containing protein complex remains to be established. | 24115750
 |
cAMP-stimulated phosphorylation of diaphanous 1 regulates protein stability and interaction with binding partners in adrenocortical cells.
Li, D; Dammer, EB; Lucki, NC; Sewer, MB
Mol Biol Cell
24
848-57
2013
概要を表示する
Diaphanous homologue 1 (DIAPH1) is a Rho effector protein that coordinates cellular dynamics by regulating microfilament and microtubule function. We previously showed that DIAPH1 plays an integral role in regulating the production of cortisol by controlling the rate of mitochondrial movement, by which activation of the adrenocorticotropin (ACTH)/cAMP signaling pathway stimulates mitochondrial trafficking and promotes the interaction between RhoA and DIAPH1. In the present study we use mass spectrometry to identify DIAPH1 binding partners and find that DIAPH1 interacts with several proteins, including RhoA, dynamin-1, kinesin, β-tubulin, β-actin, oxysterol-binding protein (OSBP)-related protein 2 (ORP2), and ORP10. Moreover, DIAPH1 is phosphorylated in response to dibutyryl cAMP (Bt2cAMP) at Thr-759 via a pathway that requires extracellular signal-related kinase (ERK). Alanine substitution of Thr-759 renders DIAPH1 more stable and attenuates the interaction between DIAPH1 and kinesin, ORP2, and actin but has no effect on the ability of the protein to interact with RhoA or β-tubulin. Finally, overexpression of a DIAPH1 T759A mutant significantly decreases the rate of Bt2cAMP-stimulated mitochondrial movement. Taken together, our findings establish a key role for phosphorylation in regulating the stability and function of DIAPH1. | 23325789
 |
Visualization and biochemical analyses of the emerging mammalian 14-3-3-phosphoproteome.
Johnson, C; Tinti, M; Wood, NT; Campbell, DG; Toth, R; Dubois, F; Geraghty, KM; Wong, BH; Brown, LJ; Tyler, J; Gernez, A; Chen, S; Synowsky, S; MacKintosh, C
Molecular & cellular proteomics : MCP
10
M110.005751
2011
概要を表示する
Hundreds of candidate 14-3-3-binding (phospho)proteins have been reported in publications that describe one interaction at a time, as well as high-throughput 14-3-3-affinity and mass spectrometry-based studies. Here, we transcribed these data into a common format, deposited the collated data from low-throughput studies in MINT (http://mint.bio.uniroma2.it/mint), and compared the low- and high-throughput data in VisANT graphs that are easy to analyze and extend. Exploring the graphs prompted questions about technical and biological specificity, which were addressed experimentally, resulting in identification of phosphorylated 14-3-3-binding sites in the mitochondrial import sequence of the iron-sulfur cluster assembly enzyme (ISCU), cytoplasmic domains of the mitochondrial fission factor (MFF), and endoplasmic reticulum-tethered receptor expression-enhancing protein 4 (REEP4), RNA regulator SMAUG2, and cytoskeletal regulatory proteins, namely debrin-like protein (DBNL) and kinesin light chain (KLC) isoforms. Therefore, 14-3-3s undergo physiological interactions with proteins that are destined for diverse subcellular locations. Graphing and validating interactions underpins efforts to use 14-3-3-phosphoproteomics to identify mechanisms and biomarkers for signaling pathways in health and disease. | 21725060
 |
Kinesin and kinectin can associate with the melanosomal surface and form a link with microtubules in normal human melanocytes.
Vancoillie, G, et al.
J. Invest. Dermatol., 114: 421-9 (2000)
2000
概要を表示する
Microtubuli play an important role in the organization of organelles and membrane traffic. They are present in melanocytic dendrites through which melanosomes are transported towards keratinocytes. Besides the actin-based motility systems, microtubuli-associated motor proteins also play a critical role in melanosome movement, as has recently been confirmed in mouse melanocytes. We investigated the in vitro expression of two forms of human conventional kinesin and its receptor kinectin in normal human epidermal melanocytes, keratinocytes, and dermal fibroblasts by reverse transcription polymerase chain reaction and northern blot analysis. In an attempt to gain insight into the subcellular distribution of kinesin and kinectin in melanocytes, double immunofluorescent staining and immunogold electron microscopy were performed. In all studied skin cells ubiquitous and neuronal kinesin are expressed, as well as the kinectin receptor. Immunofluorescent staining shows distinct but partially overlapping distributions for kinesin heavy chain and melanosomes, suggesting that kinesin is associated with some but not all of the melanosomes. Similar observations for kinectin indicate that this receptor can colocalize with melanosomes, which was confirmed by immunoelectron microscopy. The latter technique allowed us to demonstrate a close association between kinesin heavy chain, microtubuli, and melanosomes. The combined data from reverse transcription polymerase chain reaction, northern blot analysis, double immunofluorescent staining, and immunogold electron microscopy suggest that kinesins and kinectin have an important role in microtubuli-based melanosome transport in human melanocytes. | 10692099
 |
Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic.
Lippincott-Schwartz, J, et al.
J. Cell Biol., 128: 293-306 (1995)
1995
概要を表示する
The distribution and dynamics of both the ER and Golgi complex in animal cells are known to be dependent on microtubules; in many cell types the ER extends toward the plus ends of microtubules at the cell periphery and the Golgi clusters at the minus ends of microtubules near the centrosome. In this study we provide evidence that the microtubule motor, kinesin, is present on membranes cycling between the ER and Golgi and powers peripherally directed movements of membrane within this system. Immunolocalization of kinesin at both the light and electron microscopy levels in NRK cells using the H1 monoclonal antibody to kinesin heavy chain, revealed kinesin to be associated with all membranes of the ER/Golgi system. At steady-state at 37 degrees C, however, kinesin was most concentrated on peripherally distributed, pre-Golgi structures containing beta COP and vesicular stomatitis virus glycoprotein newly released from the ER. Upon temperature reduction or nocodazole treatment, kinesin's distribution shifted onto the Golgi, while with brefeldin A (BFA)-treatment, kinesin could be found in both Golgi-derived tubules and in the ER. This suggested that kinesin associates with membranes that constitutively cycle between the ER and Golgi. Kinesin's role on these membranes was examined by microinjecting kinesin antibody. Golgi-to-ER but not ER-to-Golgi membrane transport was found to be inhibited by the microinjected anti-kinesin, suggesting kinesin powers the microtubule plus end-directed recycling of membrane to the ER, and remains inactive on pre-Golgi intermediates that move toward the Golgi complex. | 7844144
 |
Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration.
Hirokawa, N, et al.
Cell, 56: 867-78 (1989)
1989
概要を表示する
Kinesin is a microtubule-activated ATPase thought to transport membrane-bounded organelles along MTs. To illuminate the structural basis for this function, EM was used to locate submolecular domains on bovine brain kinesin. Rotary shadowed kinesin appeared rod-shaped and approximately 80 nm long. One end of each molecule contained a pair of approximately 10 x 9 nm globular domains, while the opposite end was fan-shaped. Monoclonal antibodies against the approximately 124 kd heavy chains of kinesin decorated the globular structures, while those specific for the approximately 64 kd light chains labeled the fan-shaped end. Quick-freeze, deep-etch EM was used to analyze MTs polymerized from tubulin and cross-linked to latex microspheres by kinesin. Microspheres frequently attached to MTs by arm-like structures, 25-30 nm long. The MT attachment sites often appeared as one or two approximately 10 nm globular bulges. Morphologically similar cross-links were observed by quick-freeze, deep-etch EM between organelles and MTs in the neuronal cytoskeleton in vivo. These collective observations suggest that bovine brain kinesin binds to MTs by globular domains that contain the heavy chains, and that the attachment sites for organelles are at the opposite, fan-shaped end of kinesin, where the light chains are located. | 2522351
 |
Monoclonal antibodies to kinesin heavy and light chains stain vesicle-like structures, but not microtubules, in cultured cells.
Pfister, K K, et al.
J. Cell Biol., 108: 1453-63 (1989)
1989
概要を表示する
Kinesin, a microtubule-activated ATPase and putative motor protein for the transport of membrane-bounded organelles along microtubules, was purified from bovine brain and used as an immunogen for the production of murine monoclonal antibodies. Hybridoma lines that secreted five distinct antikinesin IgGs were cloned. Three of the antibodies reacted on immunoblots with the 124-kD heavy chain of kinesin, while the other two antibodies recognized the 64-kD light chain. When used for immunofluorescence microscopy, the antibodies stained punctate, cytoplasmic structures in a variety of cultured mammalian cell types. Consistent with the identification of these structures as membrane-bounded organelles was the observation that cells which had been extracted with Triton X-100 before fixation contained little or no immunoreactive material. Staining of microtubules in the interphase cytoplasm or mitotic spindle was never observed, nor were associated structures, such as centrosomes and primary cilia, labeled by any of the antibodies. Nevertheless, in double-labeling experiments using antibodies to kinesin and tubulin, kinesin-containing particles were most abundant in regions where microtubules were most highly concentrated and the particles often appeared to be aligned on microtubules. These results constitute the first direct evidence for the association of kinesin with membrane-bounded organelles, and suggest a molecular mechanism for organelle motility based on transient interactions of organelle-bound kinesin with the microtubule surface. | 2522455
 |