Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits.
Bader, Verian, et al.
Hum. Mol. Genet., 21: 4406-18 (2012)
2012
概要を表示する
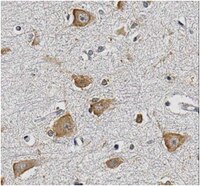
Schizophrenia is a chronic illness of heterogenous biological origin. We hypothesized that, similar to chronic progressive brain conditions, persistent functional disturbances of neurons would result in disturbed proteostasis in the brains of schizophrenia patients, leading to increased abundance of specific misfolded, insoluble proteins. Identification of such proteins would facilitate the elucidation of molecular processes underlying these devastating conditions. We therefore generated antibodies against pooled insoluble proteome of post-mortem brains from schizophrenia patients in order to identify unique, disease-specific epitopes. We successfully identified such an epitope to be present on collapsin-response mediator protein 1 (CRMP1) in biochemically purified, insoluble brain fractions. A genetic association analysis for the CRMP1 gene in a large Finnish population cohort (n = 4651) corroborated the association of physical and social anhedonia with the CRMP1 locus in a DISC1 (Disrupted-in-schizophrenia 1)-dependent manner. Physical and social anhedonia are heritable traits, present as chronic, negative symptoms of schizophrenia and severe major depression, thus constituting serious vulnerability factors for mental disease. Strikingly, lymphoblastoid cell lines derived from schizophrenia patients mirrored aberrant CRMP1 immunoreactivity by showing an increase of CRMP1 expression, suggesting its potential role as a blood-based diagnostic marker. CRMP1 is a novel candidate protein for schizophrenia traits at the intersection of the reelin and DISC1 pathways that directly and functionally interacts with DISC1. We demonstrate the impact of an interdisciplinary approach where the identification of a disease-associated epitope in post-mortem brains, powered by a genetic association study, is rapidly translated into a potential blood-based diagnostic marker. | 22798627
 |
Disrupted-in-Schizophrenia-1 expression is regulated by beta-site amyloid precursor protein cleaving enzyme-1-neuregulin cascade.
Seshadri, Saurav, et al.
Proc. Natl. Acad. Sci. U.S.A., 107: 5622-7 (2010)
2010
概要を表示する
Neuregulin-1 (NRG1) and Disrupted-in-Schizophrenia-1 (DISC1) are promising susceptibility factors for schizophrenia. Both are multifunctional proteins with roles in a variety of neurodevelopmental processes, including progenitor cell proliferation, migration, and differentiation. Here, we provide evidence linking these factors together in a single pathway, which is mediated by ErbB receptors and PI3K/Akt. We show that signaling by NRG1 and NRG2, but not NRG3, increase expression of an isoform of DISC1 in vitro. Receptors ErbB2 and ErbB3, but not ErbB4, are responsible for transducing this effect, and PI3K/Akt signaling is also required. In NRG1 knockout mice, this DISC1 isoform is selectively reduced during neurodevelopment. Furthermore, a similar decrease in DISC1 expression is seen in beta-site amyloid precursor protein cleaving enzyme-1 (BACE1) knockout mice, in which NRG1/Akt signaling is reportedly impaired. In contrast to neuronal DISC1 that was reported and characterized, expression of DISC1 in other types of cells in the brain has not been addressed. Here we demonstrate that DISC1, like NRG and ErbB proteins, is expressed in neurons, astrocytes, oligodendrocytes, microglia, and radial progenitors. These findings may connect NRG1, ErbBs, Akt, and DISC1 in a common pathway, which may regulate neurodevelopment and contribute to susceptibility to schizophrenia. | 20212127
 |
Insolubility of disrupted-in-schizophrenia 1 disrupts oligomer-dependent interactions with nuclear distribution element 1 and is associated with sporadic mental disease.
Leliveld, S Rutger, et al.
J. Neurosci., 28: 3839-45 (2008)
2008
概要を表示する
Disrupted-in-schizophrenia 1 (DISC1) and other genes have been identified recently as potential molecular players in chronic psychiatric diseases such as affective disorders and schizophrenia. A molecular mechanism of how these genes may be linked to the majority of sporadic cases of these diseases remains unclear. The chronic nature and irreversibility of clinical symptoms in a subgroup of these diseases prompted us to investigate whether proteins corresponding to candidate genes displayed subtle features of protein aggregation. Here, we show that in postmortem brain samples of a distinct group of patients with phenotypes of affective disorders or schizophrenia, but not healthy controls, significant fractions of DISC1 could be identified as cold Sarkosyl-insoluble protein aggregates. A loss-of-function phenotype could be demonstrated for insoluble DISC1 through abolished binding to a key DISC1 ligand, nuclear distribution element 1 (NDEL1): in human neuroblastoma cells, DISC1 formed expression-dependent, detergent-resistant aggregates that failed to interact with endogenous NDEL1. Recombinant (r) NDEL1 expressed in Escherichia coli selectively bound an octamer of an rDISC1 fragment but not dimers or high molecular weight multimers, suggesting an oligomerization optimum for molecular interactions of DISC1 with NDEL1. For DISC1-related sporadic psychiatric disease, we propose a mechanism whereby impaired cellular control over self-association of DISC1 leads to excessive multimerization and subsequent formation of detergent-resistant aggregates, culminating in loss of ligand binding, here exemplified by NDEL1. We conclude that the absence of oligomer-dependent ligand interactions of DISC1 can be associated with sporadic mental disease of mixed phenotypes. | 18400883
 |