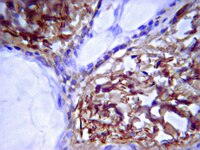
Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: clues on the mechanisms of venom-induced hemorrhage.
Herrera, C; Escalante, T; Voisin, MB; Rucavado, A; Morazán, D; Macêdo, JK; Calvete, JJ; Sanz, L; Nourshargh, S; Gutiérrez, JM; Fox, JW
PLoS neglected tropical diseases
9
e0003731
2015
概要を表示する
Snake venom hemorrhagic metalloproteinases (SVMPs) of the PI, PII and PIII classes were compared in terms of tissue localization and their ability to hydrolyze basement membrane components in vivo, as well as by a proteomics analysis of exudates collected in tissue injected with these enzymes. Immunohistochemical analyses of co-localization of these SVMPs with type IV collagen revealed that PII and PIII enzymes co-localized with type IV collagen in capillaries, arterioles and post-capillary venules to a higher extent than PI SVMP, which showed a more widespread distribution in the tissue. The patterns of hydrolysis by these three SVMPs of laminin, type VI collagen and nidogen in vivo greatly differ, whereas the three enzymes showed a similar pattern of degradation of type IV collagen, supporting the concept that hydrolysis of this component is critical for the destabilization of microvessel structure leading to hemorrhage. Proteomic analysis of wound exudate revealed similarities and differences between the action of the three SVMPs. Higher extent of proteolysis was observed for the PI enzyme regarding several extracellular matrix components and fibrinogen, whereas exudates from mice injected with PII and PIII SVMPs had higher amounts of some intracellular proteins. Our results provide novel clues for understanding the mechanisms by which SVMPs induce damage to the microvasculature and generate hemorrhage. | 25909592
 |
Three novel collagen VI chains with high homology to the alpha3 chain.
Gara, SK; Grumati, P; Urciuolo, A; Bonaldo, P; Kobbe, B; Koch, M; Paulsson, M; Wagener, R
The Journal of biological chemistry
283
10658-70
2008
概要を表示する
Here we describe three novel collagen VI chains, alpha4, alpha5, and alpha6. The corresponding genes are arranged in tandem on mouse chromosome 9. The new chains structurally resemble the collagen VI alpha3 chain. Each chain consists of seven von Willebrand factor A domains followed by a collagenous domain, two C-terminal von Willebrand factor A domains, and a unique domain. In addition, the collagen VI alpha4 chain carries a Kunitz domain at the C terminus, whereas the collagen VI alpha5 chain contains an additional von Willebrand factor A domain and a unique domain. The size of the collagenous domains and the position of the structurally important cysteine residues within these domains are identical between the collagen VI alpha3, alpha4, alpha5, and alpha6 chains. In mouse, the new chains are found in or close to basement membranes. Collagen VI alpha1 chain-deficient mice lack expression of the new collagen VI chains implicating that the new chains may substitute for the alpha3 chain, probably forming alpha1alpha2alpha4, alpha1alpha2alpha5, or alpha1alpha2alpha6 heterotrimers. Due to a large scale pericentric inversion, the human COL6A4 gene on chromosome 3 was broken into two pieces and became a non-processed pseudogene. Recently COL6A5 was linked to atopic dermatitis and designated COL29A1. The identification of novel collagen VI chains carries implications for the etiology of atopic dermatitis as well as Bethlem myopathy and Ullrich congenital muscular dystrophy. | 18276594
 |












 Antibody[MABF1580_FC CD3e (Mouse) Antibody-ALL].jpg)