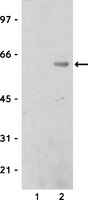
Dynactin integrity depends upon direct binding of dynamitin to Arp1.
Cheong, FK; Feng, L; Sarkeshik, A; Yates, JR; Schroer, TA
Molecular biology of the cell
25
2171-80
2014
概要を表示する
Dynactin is a multiprotein complex that works with cytoplasmic dynein and other motors to support a wide range of cell functions. It serves as an adaptor that binds both dynein and cargoes and enhances single-motor processivity. The dynactin subunit dynamitin (also known as p50) is believed to be integral to dynactin structure because free dynamitin displaces the dynein-binding p150(Glued) subunit from the cargo-binding Arp1 filament. We show here that the intrinsically disordered dynamitin N-terminus binds to Arp1 directly. When expressed in cells, dynamitin amino acids (AA) 1-87 causes complete release of endogenous dynamitin, p150, and p24 from dynactin, leaving behind Arp1 filaments carrying the remaining dynactin subunits (CapZ, p62, Arp11, p27, and p25). Tandem-affinity purification-tagged dynamitin AA 1-87 binds the Arp filament specifically, and binding studies with purified native Arp1 reveal that this fragment binds Arp1 directly. Neither CapZ nor the p27/p25 dimer contributes to interactions between dynamitin and the Arp filament. This work demonstrates for the first time that Arp1 can directly bind any protein besides another Arp and provides important new insight into the underpinnings of dynactin structure. | | 24829381
 |
Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP.
Liao, KC; Mogridge, J
Infection and immunity
81
570-9
2013
概要を表示する
The efficacy of the innate immune system depends on its ability to mount an appropriate response to diverse infections and damaging agents. Key components of this system are pattern recognition receptors that detect pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs). Nlrp1b is a pattern recognition receptor that forms a caspase-1 activation platform, known as an inflammasome, upon sensing the proteolytic activity of anthrax lethal toxin. The activation of caspase-1 leads to the release of proinflammatory cytokines that aid in the clearance of the anthrax infection. Here, we demonstrate that Nlrp1b also becomes activated in cells that are subjected to energy stress caused by metabolic inhibitors or by nutrient deprivation. Glucose starvation and hypoxia were used to correlate the level of cytosolic ATP to the degree of inflammasome activation. Because lowering the ratio of cytosolic ATP to AMP activates the main cellular energy sensor, AMP-activated protein kinase (AMPK), we assessed whether AMPK promoted inflammasome activity by using a combination of small interfering RNA (siRNA) and transfection of a dominant negative AMPK subunit. We found that AMPK promoted inflammasome activity, but activation of AMPK in the absence of ATP depletion was not sufficient for caspase-1-mediated pro-interleukin 1β (pro-IL-1β) processing. Finally, we found that mutation of the ATP-binding motif of Nlrp1b caused constitutive activation, suggesting that ATP might inhibit the Nlrp1b inflammasome instead of being required for its assembly. | Western Blotting | 23230290
 |
Proteolytic processing of Nlrp1b is required for inflammasome activity.
Frew, BC; Joag, VR; Mogridge, J
PLoS pathogens
8
e1002659
2012
概要を表示する
Nlrp1b is a NOD-like receptor that detects the catalytic activity of anthrax lethal toxin and subsequently co-oligomerizes into a pro-caspase-1 activation platform known as an inflammasome. Nlrp1b has two domains that promote oligomerization: a NACHT domain, which is a member of the AAA+ ATPase family, and a poorly characterized Function to Find Domain (FIIND). Here we demonstrate that proteolytic processing within the FIIND generates N-terminal and C-terminal cleavage products of Nlrp1b that remain associated in both the auto-inhibited state and in the activated state after cells have been treated with lethal toxin. Functional significance of cleavage was suggested by the finding that mutations that block processing of Nlrp1b also prevent the ability of Nlrp1b to activate pro-caspase-1. By using an uncleaved mutant of Nlrp1b, we established the importance of cleavage by inserting a heterologous TEV protease site into the FIIND and demonstrating that TEV protease processed this site and induced inflammasome activity. Proteolysis of Nlrp1b was shown to be required for the assembly of a functional inflammasome: a mutation within the FIIND that abolished cleavage had no effect on self-association of a FIIND-CARD fragment, but did reduce the recruitment of pro-caspase-1. Our work indicates that a post-translational modification enables Nlrp1b to function. | | 22536155
 |
Development of the "Three-step MACS": a novel strategy for isolating rare cell populations in the absence of known cell surface markers from complex animal tissue.
Lee, MY; Lufkin, T
Journal of biomolecular techniques : JBT
23
69-77
2012
概要を表示する
To circumvent the difficulty of isolating specific cell populations by MACS from dissociated complex animal tissue, when their proportions reached levels similar to that of the background, we developed the "Three-step MACS" strategy. Cells of interest are defined by their expression of a particular gene(s) of interest rather by than their natural cell surface markers or size. A two-component transgenic cell surface protein, for two sequential rounds of MACS, is expressed under the promoter control of the endogenous gene of interest by means of gene targeting and the generation of transgenic tissue. An initial step to remove dead cells is also used. Here, we describe proof-of-concept experiments, using the biotin acceptor peptide (BAP)-low-affinity nerve growth factor receptor as the two-component protein. The first component, the BAP, can be biotinylated in specific subsets of cells expressing a particular gene by expressing the biotinylating enzyme, hBirA = humanized BirA (hBirA), under the promoter control of another gene defining the specific subpopulation. We showed that a rare population of cells (1.1% of the 13.5 days postcoital mouse embryo) could be enriched to a sufficiently high purity (84.4%). From another sample with 0.1% of our cells of interest, we achieved a 40.3% pure sample. The low cost, speed, and technical ease of the Three-step MACS also make it scalable and hence, an ideal method for preparing sufficient quantities of biological samples for sensitive, high-throughput assays. | Flow Cytometry | 22951961
 |
Role of km23-1 in RhoA/actin-based cell migration.
Jin, Q; Pulipati, NR; Zhou, W; Staub, CM; Liotta, LA; Mulder, KM
Biochemical and biophysical research communications
428
333-8
2012
概要を表示する
km23-1 was originally identified as a TGFß receptor-interacting protein that plays an important role in TGFß signaling. Moreover, km23-1 is actually part of an ancient superfamily of NTPase-regulatory proteins, widely represented in archaea and bacteria. To further elucidate the function of km23-1, we identified novel protein interacting partners for km23-1 by using tandem affinity purification (TAP) and tandem mass spectrometry (MS). Here we show that km23-1 interacted with a class of proteins involved in actin-based cell motility and modulation of the actin cytoskeleton. We further showed that km23-1 modulates the formation of a highly organized stress fiber network. More significantly, we demonstrated that knockdown (KD) of km23-1 decreased RhoA activation in Mv1Lu epithelial cells. Finally, our results demonstrated for the first time that depletion of km23-1 inhibited cell migration of human colon carcinoma cells (HCCCs) in wound-healing assays. Overall, our findings demonstrate that km23-1 regulates RhoA and motility-associated actin modulating proteins, suggesting that km23-1 may represent a novel target for anti-metastatic therapy. | | 23079622
 |
SWI/SNF and Asf1 independently promote derepression of the DNA damage response genes under conditions of replication stress.
Minard, LV; Lin, LJ; Schultz, MC
PloS one
6
e21633
2011
概要を表示する
The histone chaperone Asf1 and the chromatin remodeler SWI/SNF have been separately implicated in derepression of the DNA damage response (DDR) genes in yeast cells treated with genotoxins that cause replication interference. Using genetic and biochemical approaches, we have tested if derepression of the DDR genes in budding yeast involves functional interplay between Asf1 and SWI/SNF. We find that Asf1 and SWI/SNF are both recruited to DDR genes under replication stress triggered by hydroxyurea, and have detected a soluble complex that contains Asf1 and the Snf2 subunit of SWI/SNF. SWI/SNF recruitment to DDR genes however does not require Asf1, and deletion of Snf2 does not affect Asf1 occupancy of DDR gene promoters. A checkpoint engagement defect is sufficient to explain the synthetic effect of deletion of ASF1 and SNF2 on derepression of the DDR genes in hydroxyurea-treated cells. Collectively, our results show that the DDR genes fall into a class in which Asf1 and SWI/SNF independently control transcriptional induction. | | 21738741
 |
Asf1 can promote trimethylation of H3 K36 by Set2.
Lin, LJ; Minard, LV; Johnston, GC; Singer, RA; Schultz, MC
Molecular and cellular biology
30
1116-29
2010
概要を表示する
Asf1 is a conserved histone H3/H4 chaperone that can assemble and disassemble nucleosomes and promote histone acetylation. Set2 is an H3 K36 methyltransferase. The functions of these proteins intersect in the context of transcription elongation by RNA polymerase II: both contribute to the establishment of repressive chromatin structures that inhibit spurious intragenic transcription. Here we characterize further interactions between budding yeast (Saccharomyces cerevisiae) Asf1 and Set2 using assays of intragenic transcription, H3/H4 posttranslational modification, coding region cross-linking of Asf1 and Set2, and cooccurrence of Asf1 and Set2 in protein complexes. We find that at some genes Asf1 and Set2 control chromatin metabolism as components of separate pathways. However, the existence of a low-abundance complex containing both proteins suggests that Asf1 and Set2 can more directly collaborate in chromatin regulation. Consistent with this possibility, we show that Asf1 stimulates Set2 occupancy of the coding region of a highly transcribed gene by a mechanism that depends on Asf1 binding to H3/H4. This function of Asf1 promotes the switch from di- to trimethylation of H3 K36 at that gene. These results support the view that Set2 function in chromatin metabolism can intimately involve histone chaperone Asf1. 記事全文 | | 20048053
 |
Mutational analysis of the C-terminal FATC domain of Saccharomyces cerevisiae Tra1.
Hoke, SM; Irina Mutiu, A; Genereaux, J; Kvas, S; Buck, M; Yu, M; Gloor, GB; Brandl, CJ
Current genetics
56
447-65
2010
概要を表示する
Tra1 is a component of the Saccharomyces cerevisiae SAGA and NuA4 complexes and a member of the PIKK family, which contain a C-terminal phosphatidylinositol 3-kinase-like (PI3K) domain followed by a 35-residue FATC domain. Single residue changes of L3733A and F3744A, within the FATC domain, resulted in transcriptional changes and phenotypes that were similar but not identical to those caused by mutations in the PI3K domain or deletions of other SAGA or NuA4 components. The distinct nature of the FATC mutations was also apparent from the additive effect of tra1-L3733A with SAGA, NuA4, and tra1 PI3K domain mutations. Tra1-L3733A associates with SAGA and NuA4 components and with the Gal4 activation domain, to the same extent as wild-type Tra1; however, steady-state levels of Tra1-L3733A were reduced. We suggest that decreased stability of Tra1-L3733A accounts for the phenotypes since intragenic suppressors of tra1-L3733A restored Tra1 levels, and reducing wild-type Tra1 led to comparable growth defects. Also supporting a key role for the FATC domain in the structure/function of Tra1, addition of a C-terminal glycine residue resulted in decreased association with Spt7 and Esa1, and loss of cellular viability. These findings demonstrate the regulatory potential of mechanisms targeting the FATC domains of PIKK proteins. 記事全文 | Western Blotting | 20635087
 |
Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin.
Liao, KC; Mogridge, J
Infection and immunity
77
4455-62
2009
概要を表示する
Anthrax lethal toxin causes macrophages and dendritic cells from some mouse strains to undergo caspase-1-dependent cell death. Central to this process is the NOD-like receptor Nlrp1b (Nalp1b), which detects intoxication and then self-associates to form a complex, termed an inflammasome, that is capable of activating the procaspase-1 zymogen. The nature of the signal detected directly by Nlrp1b is not known, and the mechanisms of inflammasome assembly are poorly understood. Here, we demonstrate that transfection of human fibroblasts with plasmids encoding murine Nlrp1b and procaspase-1 was sufficient to confer susceptibility to lethal toxin-mediated death on the cells. As has been observed in murine macrophages, the enzymatic activities of lethal toxin and the proteasome were both required for activation of the Nlrp1b inflammasome and this activation led to prointerleukin-1 beta processing. Release of interleukin-1beta from cells was not dependent on cell lysis, as its secretion was not affected by an osmoprotectant that prevented the appearance of lactate dehydrogenase in the culture medium. We generated constitutively active mutants of Nlrp1b by making amino-terminal deletions to the protein and observed that the ability to activate procaspase-1 was dependent on the CARD domain, which bound procaspase-1, and a region adjacent to the CARD domain that promoted self-association. Our results demonstrate that lethal toxin can activate Nlrp1b in a nonmyeloid cell line and are consistent with work that suggests that activation induces proximity of procaspase-1. | Western Blotting | 19651869
 |
A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA.
Friis, RM; Wu, BP; Reinke, SN; Hockman, DJ; Sykes, BD; Schultz, MC
Nucleic acids research
37
3969-80
2009
概要を表示する
Little is known about what enzyme complexes or mechanisms control global lysine acetylation in the amino-terminal tails of the histones. Here, we show that glucose induces overall acetylation of H3 K9, 18, 27 and H4 K5, 8, 12 in quiescent yeast cells mainly by stimulating two KATs, Gcn5 and Esa1. Genetic and pharmacological perturbation of carbon metabolism, combined with (1)H-NMR metabolic profiling, revealed that glucose induction of KAT activity directly depends on increased glucose catabolism. Glucose-inducible Esa1 and Gcn5 activities predominantly reside in the picNuA4 and SAGA complexes, respectively, and act on chromatin by an untargeted mechanism. We conclude that direct metabolic regulation of globally acting KATs can be a potent driving force for reconfiguration of overall histone acetylation in response to a physiological cue. 記事全文 | | 19406923
 |