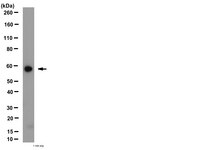
Conditional overexpression of TGFβ1 promotes pulmonary inflammation, apoptosis and mortality via TGFβR2 in the developing mouse lung.
Sureshbabu, A; Syed, MA; Boddupalli, CS; Dhodapkar, MV; Homer, RJ; Minoo, P; Bhandari, V
Respiratory research
16
4
2015
概要を表示する
Earlier studies have reported that transforming growth factor beta 1(TGFβ1) is a critical mediator of hyperoxia-induced acute lung injury (HALI) in developing lungs, leading to impaired alveolarization and a pulmonary phenotype of bronchopulmonary dysplasia (BPD). However, the mechanisms responsible for the TGFβ1-induced inflammatory signals that lead to cell death and abnormal alveolarization are poorly understood. We hypothesized that TGFβ1 signaling via TGFβR2 is necessary for the pathogenesis of the BPD pulmonary phenotype resulting from HALI.We utilized lung epithelial cell-specific TGFβ1 overexpressing transgenic and TGFβR2 null mutant mice to evaluate the effects on neonatal mortality as well as pulmonary inflammation and apoptosis in developing lungs. Lung morphometry was performed to determine the impaired alveolarization and multicolor flow cytometry studies were performed to detect inflammatory macrophages and monocytes in lungs. Apoptotic cell death was measured with TUNEL assay, immunohistochemistry and western blotting and protein expression of angiogenic mediators were also analyzed.Our data reveals that increased TGFβ1 expression in newborn mice lungs leads to increased mortality, macrophage and immature monocyte infiltration, apoptotic cell death specifically in Type II alveolar epithelial cells (AECs), impaired alveolarization, and dysregulated angiogenic molecular markers.Our study has demonstrated the potential role of inhibition of TGFβ1 signaling via TGFβR2 for improved survival, reduced inflammation and apoptosis that may provide insights for the development of potential therapeutic strategies targeted against HALI and BPD. | Immunohistochemistry | 25591994
 |
Effects of angiopoietin-1 on hemorrhagic transformation and cerebral edema after tissue plasminogen activator treatment for ischemic stroke in rats.
Kawamura, K; Takahashi, T; Kanazawa, M; Igarashi, H; Nakada, T; Nishizawa, M; Shimohata, T
PloS one
9
e98639
2014
概要を表示する
An angiogenesis factor, angiopoietin-1 (Ang1), is associated with the blood-brain barrier (BBB) disruption after focal cerebral ischemia. However, whether hemorrhagic transformation and cerebral edema after tissue plasminogen activator (tPA) treatment are related to the decrease in Ang1 expression in the BBB remains unknown. We hypothesized that administering Ang1 might attenuate hemorrhagic transformation and cerebral edema after tPA treatment by stabilizing blood vessels and inhibiting hyperpermeability. Sprague-Dawley rats subjected to thromboembolic focal cerebral ischemia were assigned to a permanent ischemia group (permanent middle cerebral artery occlusion; PMCAO) and groups treated with tPA at 1 h or 4 h after ischemia. Endogenous Ang1 expression was observed in pericytes, astrocytes, and neuronal cells. Western blot analyses revealed that Ang1 expression levels on the ischemic side of the cerebral cortex were decreased in the tPA-1h, tPA-4h, and PMCAO groups as compared to those in the control group (P = 0.014, 0.003, and 0.014, respectively). Ang1-positive vessel densities in the tPA-4h and PMCAO groups were less than that in the control group (p = 0.002 and less than 0.001, respectively) as well as that in the tPA-1h group (p = 0.047 and 0.005, respectively). These results suggest that Ang1-positive vessel density was maintained when tPA was administered within the therapeutic time window (1 h), while it was decreased when tPA treatment was given after the therapeutic time window (4 h). Administering Ang1 fused with cartilage oligomeric protein (COMP) to supplement this decrease has the potential to suppress hemorrhagic transformation as measured by hemoglobin content in a whole cerebral homogenate (p = 0.007) and cerebral edema due to BBB damage (p = 0.038), as compared to administering COMP protein alone. In conclusion, Ang1 might be a promising target molecule for developing vasoprotective therapies for controlling hemorrhagic transformation and cerebral edema after tPA treatment. | Western Blotting | 24896569
 |