Distinct Localization of Mature HGF from its Precursor Form in Developing and Repairing the Stomach
Nawaphat Jangphattananont 1 , Hiroki Sato 2 3 , Ryu Imamura 4 5 , Katsuya Sakai 6 7 , Yumi Terakado 8 , Kazuhiro Murakami 9 , Nick Barker 10 11 , Hiroko Oshima 12 13 , Masanobu Oshima 14 15 , Junichi Takagi 16 , Yukinari Kato 17 , Seiji Yano 18 19 , Kunio Matsumoto
Int J Mol Sci
20(12)
2955
2019
概要を表示する
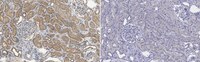
Hepatocyte growth factor (HGF) is secreted as an inactive single-chain HGF (scHGF); however, only proteolytically processed two-chain HGF (tcHGF) can activate the MET receptor. We investigated the localization of tcHGF and activated/phosphorylated MET (pMET) using a tcHGF-specific antibody. In day 16.5 mouse embryos, total HGF (scHGF + tcHGF) was mainly localized in smooth muscle cells close to, but separate from, MET-positive epithelial cells in endodermal organs, including the stomach. In the adult stomach, total HGF was localized in smooth muscle cells, and tcHGF was mainly localized in the glandular base region. Immunostaining for pMET and Lgr5-driven green fluorescent protein (GFP) indicated that pMET localization overlapped with Lgr5+ gastric stem cells. HGF promoted organoid formation similar to EGF, indicating the potential for HGF to promote the survival and growth of gastric stem cells. pMET and tcHGF localizations changed during regeneration following gastric injury. These results indicate that MET is constantly activated in gastric stem cells and that the localization of pMET differs from the primary localization of precursor HGF but has a close relationship to tcHGF. Our results suggest the importance of the microenvironmental generation of tcHGF in the regulation of development, regeneration, and stem cell behavior. | 31212972
 |
Probing conformational and functional states of human hepatocyte growth factor by a panel of monoclonal antibodies
Masataka Umitsu 1 , Katsuya Sakai 2 , Satoshi Ogasawara 3 , Mika K Kaneko 3 , Ryoko Asaki 1 , Keiko Tamura-Kawakami 1 , Yukinari Kato 3 , Kunio Matsumoto 2 , Junichi Takagi
Sci Rep
6
33149
2016
概要を表示する
HGF-Met signaling contributes to various biological events by controlling cell migration. Since the abnormal activation of Met receptor causes cancer progression, inhibitors such as neutralizing antibodies are regarded as promising therapeutics. HGF is secreted as a single-chain (sc) precursor and is processed by extracellular proteases to generate disulfide-bonded two-chain (tc) HGF. Although this proteolytic processing of HGF is necessary for its biological activity, exactly how the proteolysis leads to the conversion of HGF to the active form is still unclar due to the lack of structural information. In order to gain insights about this point, we generated 6 antibodies against HGF. All antibodies recognized different epitopes on the native HGF protein and showed distinct effects when tested in a cell-based HGF-Met signaling assay. They included one antibody (t1E4) that strongly blocks Met activation by tcHGF, as well as one antibody (t8E4) exclusively recognizing the active tcHGF but not inactive scHGF. Thus, a panel of anti-HGF antibodies suitable for probing the structural mechanism of HGF activation were obtained. | 27608665
 |