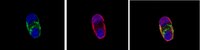
A phenotypic culture system for the molecular analysis of CNS myelination in the spinal cord.
Davis, H; Gonzalez, M; Stancescu, M; Love, R; Hickman, JJ; Lambert, S
Biomaterials
35
8840-5
2014
概要を表示する
Studies of central nervous system myelination lack defined in vitro models which would effectively dissect molecular mechanisms of myelination that contain cells of the correct phenotype. Here we describe a co-culture of purified motoneurons and oligodendrocyte progenitor cells, isolated from rat embryonic spinal cord using a combination of immunopanning techniques. This model illustrates differentiation of oligodendrocyte progenitors into fully functional mature oligodendrocytes that myelinate axons. It also illustrates a contribution of axons to the rate of oligodendrocyte maturation and myelin gene expression. The defined conditions used allow molecular analysis of distinct stages of myelination and precise manipulation of inductive cues affecting axonal-oligodendrocyte interactions. This phenotypic in vitro myelination model can provide valuable insight into our understanding of demyelinating disorders, such as multiple sclerosis and traumatic diseases such as spinal cord injury where demyelination represents a contributing factor to the pathology of the disorder. | | | 25064806
 |
Analysis of human embryonic stem cells with regulatable expression of the cell adhesion molecule l1 in regeneration after spinal cord injury.
Yoo, M; Lee, GA; Park, C; Cohen, RI; Schachner, M
Journal of neurotrauma
31
553-64
2014
概要を表示する
Cell replacement therapy is one potential avenue for central nervous system (CNS) repair. However, transplanted stem cells may not contribute to long-term recovery of the damaged CNS unless they are engineered for functional advantage. To fine tune regenerative capabilities, we developed a human neural cell line expressing L1, a regeneration-conducive adhesion molecule, under the control of a doxycycline regulatable Tet-off promoter. Controlled expression of L1 is desired because overexpression after regenerative events may lead to adverse consequences. The regulated system was tested in several cell lines, where doxycycline completely eliminated green fluorescent protein or L1 expression by 3-5 days in vitro. Increased colony formation as well as decreased proliferation were observed in H9NSCs without doxycycline (hL1-on). To test the role of L1 in vivo after acute compression spinal cord injury of immunosuppressed mice, quantum dot labeled hL1-on or hL1-off cells were injected at three sites: lesion; proximal; and caudal. Mice transplanted with hL1-on cells showed a better Basso Mouse Scale score, when compared to those with hL1-off cells. As compared to the hL1-off versus hL1-on cell transplanted mice 6 weeks post-transplantation, expression levels of L1, migration of transplanted cells, and immunoreactivity for tyrosine hydroxylase were higher, whereas expression of chondroitin sulfate proteoglycans was lower. Results indicate that L1 expression is regulatable in human stem cells by doxycycline in a nonviral engineering approach. Regulatable expression in a prospective nonleaky Tet-off system could hold promise for therapy, based on the multifunctional roles of L1, including neuronal migration and survival, neuritogenesis, myelination, and synaptic plasticity. | | | 24125017
 |
Strategies for repair of white matter: influence of osmolarity and microglia on proliferation and apoptosis of oligodendrocyte precursor cells in different basal culture media.
Kleinsimlinghaus, K; Marx, R; Serdar, M; Bendix, I; Dietzel, ID
Frontiers in cellular neuroscience
7
277
2013
概要を表示する
The aim of the present study has been to obtain high yields of oligodendrocyte precursor cells (OPCs) in culture. This is a first step in facilitation of myelin repair. We show that, in addition to factors, known to promote proliferation, such as basic fibroblast growth factor (FGF-2) and platelet derived growth factor (PDGF) the choice of the basal medium exerts a significant influence on the yield of OPCs in cultures from newborn rats. During a culture period of up to 9 days we observed larger numbers of surviving cells in Dulbecco's Modified Eagle Medium (DMEM), and Roswell Park Memorial Institute Medium (RPMI) compared with Neurobasal Medium (NB). A larger number of A2B5-positive OPCs was found after 6 days in RPMI based media compared with NB. The percentage of bromodeoxyuridine (BrdU)-positive cells was largest in cultures maintained in DMEM and RPMI. The percentage of caspase-3 positive cells was largest in NB, suggesting that this medium inhibits OPC proliferation and favors apoptosis. A difference between NB and DMEM as well as RPMI is the reduced Na(+)-content. The addition of equiosmolar supplements of mannitol or NaCl to NB medium rescued the BrdU-incorporation rate. This suggested that the osmolarity influences the proliferation of OPCs. Plating density as well as residual microglia influence OPC survival, BrdU incorporation, and caspase-3 expression. We found, that high density cultures secrete factors that inhibit BrdU incorporation whereas the presence of additional microglia induces an increase in caspase-3 positive cells, indicative of enhanced apoptosis. An enhanced number of microglia could thus also explain the stronger inhibition of OPC differentiation observed in high density cultures in response to treatment with the cytokines TNF-α and IFN-γ. We conclude that a maximal yield of OPCs is obtained in a medium of an osmolarity higher than 280 mOsm plated at a relatively low density in the presence of as little microglia as technically achievable. | | | 24421756
 |
Alternating current electric fields of varying frequencies: effects on proliferation and differentiation of porcine neural progenitor cells.
Lim, JH; McCullen, SD; Piedrahita, JA; Loboa, EG; Olby, NJ
Cellular reprogramming
15
405-12
2013
概要を表示する
Application of sinusoidal electric fields (EFs) has been observed to affect cellular processes, including alignment, proliferation, and differentiation. In the present study, we applied low-frequency alternating current (AC) EFs to porcine neural progenitor cells (pNPCs) and investigated the effects on cell patterning, proliferation, and differentiation. pNPCs were grown directly on interdigitated electrodes (IDEs) localizing the EFs to a region accessible visually for fluorescence-based assays. Cultures of pNPCs were exposed to EFs (1 V/cm) of 1 Hz, 10 Hz, and 50 Hz for 3, 7, and 14 days and compared to control cultures. Immunocytochemistry was performed to evaluate the expression of neural markers. pNPCs grew uniformly with no evidence of alignment to the EFs and no change in cell numbers when compared with controls. Nestin expression was shown in all groups at 3 and 7 days, but not at 14 days. NG2 expression was low in all groups. Co-expression of glial fibrillary acidic protein (GFAP) and TUJ1 was significantly higher in the cultures exposed to 10- and 50-Hz EFs than the controls. In summary, sinusoidal AC EFs via IDEs did not alter the alignment and proliferation of pNPCs, but higher frequency stimulation appeared to delay differentiation into mature astrocytes. | | | 23961767
 |
hESC-derived Olig2(+) progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury.
Jiang, Peng, et al.
Nat Commun, 4: 2196 (2013)
2013
概要を表示する
Human pluripotent stem cells (hPSCs) have been differentiated to astroglia, but the utilization of hPSC-derived astroglia as cell therapy for neurological diseases has not been well studied. Astroglia are heterogeneous, and not all astroglia are equivalent in promoting neural repair. A prerequisite for cell therapy is to derive defined cell populations with superior therapeutic effects. Here we use an Olig2-GFP human embryonic stem cell (hESC) reporter to demonstrate that hESC-derived Olig2(+) progenitors generate a subtype of previously uncharacterized astroglia (Olig2PC-Astros). These Olig2PC-Astros differ substantially from astroglia differentiated from Olig2-negative hESC-derived neural progenitor cells (NPC-Astros), particularly in their neuroprotective properties. When grafted into brains subjected to global ischaemia, Olig2PC-Astros exhibit superior neuroprotective effects and improved behavioural outcome compared to NPC-Astros. Thus, this new paradigm of human astroglial differentiation is useful for studying the heterogeneity of human astroglia, and the unique Olig2PC-Astros may constitute a new cell therapy for treating cerebral ischaemia and other neurological diseases. | | | 23880652
 |
Small Molecule Induction of Human Umbilical Stem Cells into MBP-positive Oligodendrocytes in a Defined Three-Dimensional Environment.
Davis, H; Guo, X; Lambert, S; Stancescu, M; Hickman, JJ
ACS chemical neuroscience
3
31-39
2012
概要を表示する
Stem cells from umbilical cord would be a favorable alternative to embryonic stem cells for therapeutic applications. In this study, human multipotent progenitor cells (MLPCs) from umbilical cord were differentiated into oligodendrocytes by exposure to a range of microenvironmental chemical and physical cues. Chemical cues were represented by a novel defined differentiation medium containing the neurotransmitter norepinephrine (NE). In traditional 2 dimensional (2D) conditions, the MLPCs differentiated into oligodendrocyte precursors, but did not progress further. However, in a 3 dimensional (3D) environment, the MLPCs differentiated into committed oligodendrocytes that expressed MBP. The apparent method of interaction of NE in stimulating the differentiation process was identified to occur through the adenergic pathway while all prior differentiation methods have used other routes. This novel method of obtaining functional human oligodendrocytes from MLPCs would eliminate many of the difficulties associated with their differentiation from embryonic stem cells. | | | 22582139
 |
Rat Cortical Oligodendrocyte-Embryonic Motoneuron Co-Culture: An In Vitro Axon-Oligodendrocyte Interaction Model.
Davis, H; Gonzalez, M; Bhargava, N; Stancescu, M; Hickman, JJ; Lambert, S
Journal of biomaterials and tissue engineering
2
206-214
2012
概要を表示する
Mechanisms that control the differentiation and function of oligodendrocytes in the central nervous system are complex and involve multiple inputs from the surrounding environment, including localized concentrations of growth factors and the extracellular matrix. Dissection and analysis of these inputs are key to understanding the pathology of central nervous system demyelinating diseases such as multiple sclerosis, where the differentiation of myelinating oligodendrocytes from their precursors underlies the remission phase of the disease. In vitro co-culture models provide a mechanism for the study of factors that regulate differentiation of oligodendrocyte precursors but have been difficult to develop due to the complex nature of central nervous system myelination. This study describes development of an in vitro model that merges a defined medium with a chemically modified substrate to study aspects of myelination in the central nervous system. We demonstrate that oligodendrocyte precursors co-cultured with rat embryonic motoneurons on non-biological substrate (diethylenetriamine trimethoxy-silylpropyldiethylenetriamine), can be induced to differentiate into mature oligodendrocytes that express myelin basic protein, using a serum-free medium. This defined and reproducible model of in vitro myelination could be a valuable tool for the development of treatments for demyelinating diseases such as multiple sclerosis. | | | 23493660
 |
Development of a model of sacrocaudal spinal cord injury in cloned Yucatan minipigs for cellular transplantation research.
Lim, JH; Piedrahita, JA; Jackson, L; Ghashghaei, T; Olby, NJ
Cellular reprogramming
12
689-97
2010
概要を表示する
Research into transplantation strategies to treat spinal cord injury (SCI) is frequently performed in rodents, but translation of results to clinical patients can be poor and a large mammalian model of severe SCI is needed. The pig has been considered an optimal model species in which to perform preclinical testing, and the Yucatan minipig can be cloned successfully utilizing somatic cell nuclear transfer (SCNT). However, induction of paralysis in pigs poses significant welfare and nursing challenges. The present study was conducted to determine whether Yucatan SCNT clones could be used to develop an SCI animal model for cellular transplantation research. First, we demonstrated that transection of the sacrocaudal spinal cord in Yucatan SCNT clones produces profound, quantifiable neurological deficits restricted to the tail. We then established that neurospheres could be isolated from brain tissue of green fluorescence protein (GFP) transfected SCNT clones. Finally, we confirmed survival of transplanted GFP-expressing neural stem cells in the SCI lesion and their differentiation into glial and neuronal lineages for up to 4 weeks without immunosuppression. We conclude that this model of sacrocaudal SCI in Yucatan SCNT clones represents a powerful research tool to investigate the effect of cellular transplantation on axonal regeneration and functional recovery. 記事全文 | | | 21108536
 |
Generation and characterization of neurospheres from canine adipose tissue-derived stromal cells.
Ji-Hey Lim,Lindsay Boozer,Christopher L Mariani,Jorge A Piedrahita,Natasha J Olby
Cellular reprogramming
12
2010
概要を表示する
Adipose tissue-derived stromal cells (ADSCs) have been identified as a powerful stem cell source for cellular transplantation therapy. The dog is increasingly used as a model of human neurological disease; however, few studies have reported induction of canine ADSCs to neural lineages. We characterized canine ADSCs and investigated whether they could be induced to differentiate into neural lineages. Subcutaneous adipose tissue collected from the dorsal epaxial region of adult dogs aged from 1 to 6 years was cultured to produce ADSCs that were then induced to neural lineages. RT-PCR, flow cytometry, and immunocytochemistry were performed to characterize these cell populations. Morphologically fibroblast-like ADSCs were isolated and had similar characteristics to mesenchymal stem cells. Under neurogenic conditions containing basic fibroblast growth factor and epidermal growth factor, ADSCs formed spherical cellular aggregates that resembled neurospheres. RT-PCR confirmed expression of Sox2 and CD90 by these aggregates. Expression of neural stem/progenitor markers (Nestin, Sox2, Vimentin) and neural lineage markers (A2B5, GFAP, Tuj1) was shown on immunocytochemistry. After differentiation, 60% of the cells were Tuj1 positive. In conclusion, we isolated and generated neural progenitor cells from canine ADSCs. ADSCs have potential for future autologous cell transplantation therapy for neurological disorders. | | | 20698780
 |
A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure.
Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP, Devuyst O, Rampoldi L
Hum Mol Genet
19
2998-3010. Epub 2010 May 14.
2010
概要を表示する
Uromodulin-associated kidney diseases (UAKD) are autosomal-dominant disorders characterized by alteration of urinary concentrating ability, tubulo-interstitial fibrosis, hyperuricaemia and renal cysts at the cortico-medullary junction. UAKD are caused by mutations in UMOD, the gene encoding uromodulin. Although uromodulin is the most abundant protein secreted in urine, its physiological role remains elusive. Several in vitro studies demonstrated that mutations in uromodulin lead to endoplasmic reticulum (ER) retention of mutant protein, but their relevance in vivo has not been studied. We here report on the generation and characterization of the first transgenic mouse model for UAKD. Transgenic mice that express the C147W mutant uromodulin (Tg(Umod)(C147W)), corresponding to the well-established patient mutation C148W, were compared with expression-matched transgenic mice expressing the wild-type protein (Tg(Umod)(wt)). Tg(Umod)(C147W) mice recapitulate most of the UAKD features, with urinary concentrating defect of renal origin and progressive renal injury, i.e. tubulo-interstitial fibrosis with inflammatory cell infiltration, tubule dilation and specific damage of the thick ascending limb of Henle's loop, leading to mild renal failure. As observed in patients, Tg(Umod)(C147W) mice show a marked reduction of urinary uromodulin excretion. Mutant uromodulin trafficking to the plasma membrane is indeed impaired as it is retained in the ER of expressing cells leading to ER hyperplasia. The Tg(Umod)(C147W) mice represent a unique model that recapitulates most of the features associated with UAKD. Our data clearly demonstrate a gain-of-toxic function of uromodulin mutations providing insights into the pathogenetic mechanism of the disease. These findings may also be relevant for other tubulo-interstitial or ER-storage disorders. | | | 20472742
 |