324800 Sigma-AldrichEpoxomicin, Synthetic - Calbiochem
Epoxomicin, Synthetic, CAS 134381-21-8, is a potent, specific, and irreversible inhibitor of chymotrypsin-like, trypsin-like, and peptidyl-glutamyl peptide hydrolyzing activities of the proteasome.
More>> Epoxomicin, Synthetic, CAS 134381-21-8, is a potent, specific, and irreversible inhibitor of chymotrypsin-like, trypsin-like, and peptidyl-glutamyl peptide hydrolyzing activities of the proteasome. Less<<Sinonimi: Proteasome Inhibitor XIV
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| Empirical Formula |
|---|
| C₂₈H₅₀N₄O₇ |
Products
| Numero di catalogo | Confezionamento | Qtà/conf | |
|---|---|---|---|
| 324800-100UG | Bottiglia di vetro | 100 μg |
| Description | |
|---|---|
| Overview | An antitumor and anti-inflammatory agent that acts as a potent, highly specific, and irreversible inhibitor of chymotrypsin-like (CT-L), trypsin-like (T-L), and peptidyl-glutamyl peptide hydrolyzing (PGPH) activities of the proteasome. Modifies the proteasomal catalytic subunits LMP-7, MECL1, and Z. Does not affect the activities of non-proteasomal proteases such as trypsin, cathepsin B, or chymotrypsin. A 1 mM (50 µg/90 µl) solution of Epoxomicin, Synthetic (Cat. No. 324801) in DMSO is also available. |
| Catalogue Number | 324800 |
| Brand Family | Calbiochem® |
| Synonyms | Proteasome Inhibitor XIV |
| References | |
|---|---|
| References | Meng, L., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 10403. Sin, N., et al. 1999. Bioorg. Med. Chem. Lett. 9, 2283. Hanada, M., et al. 1992. J. Antibiot. 45, 1746. |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White solid |
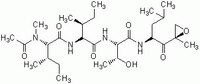
| Hill Formula | C₂₈H₅₀N₄O₇ |
| Chemical formula | C₂₈H₅₀N₄O₇ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | CT-L,T-L,PGPH activity of proteasome |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 324800-100UG | 04055977216158 |
Documentation
Epoxomicin, Synthetic - Calbiochem MSDS
| Titolo |
|---|
Epoxomicin, Synthetic - Calbiochem Certificati d'Analisi
| Titolo | Numero di lotto |
|---|---|
| 324800 |
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Meng, L., et al. 1999. Proc. Natl. Acad. Sci. USA 96, 10403. Sin, N., et al. 1999. Bioorg. Med. Chem. Lett. 9, 2283. Hanada, M., et al. 1992. J. Antibiot. 45, 1746. |
Brochure
| Titolo |
|---|
| Caspases and other Apoptosis Related Tools Brochure |