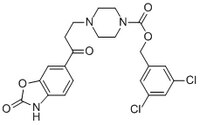
189512 Sigma-AldrichAutotaxin Inhibitor III, PF-8380 - CAS 1144035-53-9 - Calbiochem
The Autotaxin Inhibitor III, PF-8380, also referenced under CAS 1144035-53-9, controls the biological activity of Autotaxin. This small molecule/inhibitor is primarily used for Membrane applications.
More>> The Autotaxin Inhibitor III, PF-8380, also referenced under CAS 1144035-53-9, controls the biological activity of Autotaxin. This small molecule/inhibitor is primarily used for Membrane applications. Less<<Sinonimi: 6-(3-(Piperazin-1-yl)propanoyl)-benzo[d]oxazol-2(3H)-one, Atx Inhibitor III, PF-8380
Prodotti consigliati
Panoramica
| Replacement Information |
|---|
Tabella delle specifiche principali
| CAS # | Empirical Formula |
|---|---|
| 1144035-53-9 | C₂₂H₂₁Cl₂N₃O₅ |
Products
| Numero di catalogo | Confezionamento | Qtà/conf | |
|---|---|---|---|
| 189512-10MG | Bottiglia di vetro | 10 mg |
| References | |
|---|---|
| References | Gierse, J., et al. 2010. J. Pharmacol. Exp. Ther. 334, 310. |
| Product Information | |
|---|---|
| CAS number | 1144035-53-9 |
| Form | Tan powder |
| Hill Formula | C₂₂H₂₁Cl₂N₃O₅ |
| Chemical formula | C₂₂H₂₁Cl₂N₃O₅ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥95% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Numero di catalogo | GTIN |
| 189512-10MG | 04055977221886 |
Documentation
Autotaxin Inhibitor III, PF-8380 - CAS 1144035-53-9 - Calbiochem MSDS
| Titolo |
|---|
Autotaxin Inhibitor III, PF-8380 - CAS 1144035-53-9 - Calbiochem Certificati d'Analisi
| Titolo | Numero di lotto |
|---|---|
| 189512 |
Riferimenti bibliografici
| Panoramica delle referenze |
|---|
| Gierse, J., et al. 2010. J. Pharmacol. Exp. Ther. 334, 310. |