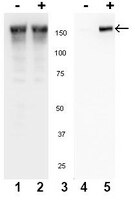
Essential role for epidermal growth factor receptor in glutamate receptor signaling to NF-kappaB.
Sitcheran, R; Comb, WC; Cogswell, PC; Baldwin, AS
Molecular and cellular biology
28
5061-70
2008
Mostra il sommario
Glutamate is a critical neurotransmitter of the central nervous system (CNS) and also an important regulator of cell survival and proliferation. The binding of glutamate to metabotropic glutamate receptors induces signal transduction cascades that lead to gene-specific transcription. The transcription factor NF-kappaB, which regulates cell proliferation and survival, is activated by glutamate; however, the glutamate receptor-induced signaling pathways that lead to this activation are not clearly defined. Here we investigate the glutamate-induced activation of NF-kappaB in glial cells of the CNS, including primary astrocytes. We show that glutamate induces phosphorylation, nuclear accumulation, DNA binding, and transcriptional activation function of glial p65. The glutamate-induced activation of NF-kappaB requires calcium-dependent IkappaB kinase alpha (IKKalpha) and IKKbeta activation and induces p65-IkappaBalpha dissociation in the absence of IkappaBalpha phosphorylation or degradation. Moreover, glutamate-induced IKK preferentially targets the phosphorylation of p65 but not IkappaBalpha. Finally, we show that the ability of glutamate to activate NF-kappaB requires cross-coupled signaling with the epidermal growth factor receptor. Our results provide insight into a glutamate-induced regulatory pathway distinct from that described for cytokine-induced NF-kappaB activation and have important implications with regard to both normal glial cell physiology and pathogenesis. | Western Blotting | 18541671
 |
Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells.
Johnson, G R, et al.
J. Biol. Chem., 268: 2924-31 (1993)
1992
Mostra il sommario
The COOH-terminal half of the amphiregulin (AR) molecule has sequence homology to epidermal growth factor (EGF). The ability of AR to elicit in vivo phosphorylation of the EGF receptor (EGFR) and p185erbB2 was studied in four human epithelial cell lines which expressed either or both of the receptor tyrosine kinases. AR induced the phosphorylation of the EGFR and p185erbB2, and phosphoamino acid analysis revealed enhanced phosphorylation of tyrosine residues in both receptor proteins. A monoclonal antibody (mAb) which binds to the extracellular domain of the EGFR blocked the phosphorylation of the EGFR and p185erbB2 as well as AR-induced mitogenesis indicating that the EGFR mediated these responses. In MDA-MB-453 cells which lack EGFRs, AR did not induce phosphorylation of p185erbB2, did not affect proliferation, and had no detectable effect on the phosphorylation of cellular proteins isolated using an anti-phosphotyrosine mAb. Qualitatively, in vivo phosphorylations induced by AR and EGF were found to be indistinguishable as demonstrated by analysis of cellular 32P-labeled proteins isolated with the anti-phosphotyrosine mAb. Moreover, in the presence of the anti-EGFR mAb, AR had no effect on the proliferation of cells. These results provide strong evidence that the EGFR is the sole cell surface mediator of the action of AR in human epithelial cells. | | 7679104
 |
Polymorphonuclear leukocytes-mediated lysis of A431 cells induced by IgG1 mouse anti-epidermal growth factor receptor monoclonal antibodies.
Kawamoto, T, et al.
In Vitro Cell. Dev. Biol., 28A: 782-6 (1992)
1992
Mostra il sommario
The ability of different Fc receptors (Fc gamma R) on IgG-mediated cytotoxicity for human epidermoid carcinoma A431 cells bearing large number of epidermal growth factor receptors (EGFRs) was examined by using two isotypes (IgG1 and IgG2a) of murine monoclonal antibodies (MoAbs) against EGFR in the presence of human monocytes and granulocytes. Two MoAbs (225 and LA1) of the IgG1 isotype exhibited effective cytolytic activity for A431 cells with human polymorphonuclear leukocytes (PMN) rather than with human mononuclear cells (MNC). In contrast, two MoAbs (528 and 579) of the IgG2a-isotype lysed the cells less effectively with PMN than with MNC. Anti-Fc gamma R II (CDw32) MoAb 2E1 inhibited the IgG1-mediated cytotoxicity by PMN, and anti-Fc gamma R III (CD16) MoAb 80H3 did not inhibit the IgG2a-mediated cytotoxicity by MNC. Under these conditions, antibody-dependent cell-mediated cytotoxicity by mouse MoAb IgG1 isotypes resulted from antibody binding to the Fc gamma R II (CDw32) of PMN. | | 1483969
 |
Cytotoxic properties of DAB486EGF and DAB389EGF, epidermal growth factor (EGF) receptor-targeted fusion toxins.
Shaw, J P, et al.
J. Biol. Chem., 266: 21118-24 (1991)
1991
Mostra il sommario
Elevated expression of the receptor for epidermal growth factor (EGF) is a characteristic of several malignancies including those of the breast, bladder, prostate, lung, and neuroglia. To therapeutically target the cytotoxic action of diphtheria toxin to EGF receptor-expressing tumor cells, we have constructed a hybrid gene in which the sequences for the binding domain of diphtheria toxin have been replaced by those for human EGF. The resulting fusion toxins, DAB486EGF and DAB389EGF, bind specifically to the EGF receptor and inhibit protein synthesis in a variety of EGF receptor expressing human tumor cell lines with an IC50 as low as 0.1 pM. Comparisons of DAB486EGF and DAB389EGF showed that DAB389EGF was consistently 10- to 100-fold more cytotoxic than DAB486EGF. Like diphtheria toxin, the cytotoxic action of DAB389EGF results from ADP-ribosylation of elongation factor-2 and is sensitive to the action of chloroquine. Studies of the kinetics of cellular intoxication showed that a 15-min exposure of EGF receptor-expressing A431 cells to DAB389EGF results in complete protein synthesis inhibition within 4 h. Furthermore, inhibition of protein synthesis results in elimination of human tumor cell colonies. These findings show that DAB389EGF is a potential therapeutic agent for a wide variety of EGF receptor-expressing solid tumors. | | 1939154
 |