AtEAF1 is a potential platform protein for Arabidopsis NuA4 acetyltransferase complex.
Bieluszewski, T; Galganski, L; Sura, W; Bieluszewska, A; Abram, M; Ludwikow, A; Ziolkowski, PA; Sadowski, J
BMC plant biology
15
75
2015
Mostra il sommario
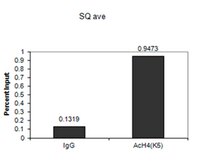
Histone acetyltransferase complex NuA4 and histone variant exchanging complex SWR1 are two chromatin modifying complexes which act cooperatively in yeast and share some intriguing structural similarities. Protein subunits of NuA4 and SWR1-C are highly conserved across eukaryotes, but form different multiprotein arrangements. For example, the human TIP60-p400 complex consists of homologues of both yeast NuA4 and SWR1-C subunits, combining subunits necessary for histone acetylation and histone variant exchange. It is currently not known what protein complexes are formed by the plant homologues of NuA4 and SWR1-C subunits.We report on the identification and molecular characterization of AtEAF1, a new subunit of Arabidopsis NuA4 complex which shows many similarities to the platform protein of the yeast NuA4 complex. AtEAF1 copurifies with Arabidopsis homologues of NuA4 and SWR1-C subunits ARP4 and SWC4 and interacts physically with AtYAF9A and AtYAF9B, homologues of the YAF9 subunit. Plants carrying a T-DNA insertion in one of the genes encoding AtEAF1 showed decreased FLC expression and early flowering, similarly to Atyaf9 mutants. Chromatin immunoprecipitation analyses of the single mutant Ateaf1b-2 and artificial miRNA knock-down Ateaf1 lines showed decreased levels of H4K5 acetylation in the promoter regions of major flowering regulator genes, further supporting the role of AtEAF1 as a subunit of the plant NuA4 complex.Growing evidence suggests that the molecular functions of the NuA4 and SWR1 complexes are conserved in plants and contribute significantly to plant development and physiology. Our work provides evidence for the existence of a yeast-like EAF1 platform protein in A. thaliana, filling an important gap in the knowledge about the subunit organization of the plant NuA4 complex. | | 25849764
 |
Differential Regulation of Vascular Endothelial Growth Factors by Promoter-targeted shRNAs.
Laham-Karam, N; Lalli, M; Leinonen, N; Ylä-Herttuala, S
Molecular therapy. Nucleic acids
4
e243
2015
Mostra il sommario
Vascular endothelial growth factors (VEGFs) and their receptors (VEGF-R) are central regulators of vasculogenesis, angiogenesis, and lymphangiogenesis. They contribute to many vascular-related pathologies, and hence VEGF-targeted therapies have been widely sought after. In this study, the authors investigated the ability of promoter-targeted small hairpin RNAs (shRNAs) to regulate VEGF-A, VEGF-C and VEGF-R1 in different cell lines. The authors identified shRNAs that can upregulate hVEGF-C at both the mRNA and protein levels, and differentially regulate hVEGF-A depending on the cell type. Likewise, the authors identified shRNA that downregulated VEGF-R1 gene expression. Hence, promoter-targeted shRNAs can affect endogenous gene expression not only bimodally, but also differentially in a cell-type specific manner. Importantly, all three genes tested were regulated by at least one shRNA, supporting the idea that nuclear RNA interference is a widespread phenomenon. The level of regulation across the panel of shRNAs varied maximally from a 2.2-fold increase to a 4-fold decrease. This level of change should be useful in fine-tuning and modulating target gene expression, which for potent molecules, such as VEGF-A and VEGF-C, can be very beneficial. These promoter-targeted shRNAs may facilitate the design and development of targeted, context-dependent strategies for both pro- and antiangiogenic therapies for the treatment of vascular-related pathologies. | | 25988242
 |
Characterization of BRD4 during mammalian postmeiotic sperm development.
Bryant, JM; Donahue, G; Wang, X; Meyer-Ficca, M; Luense, LJ; Weller, AH; Bartolomei, MS; Blobel, GA; Meyer, RG; Garcia, BA; Berger, SL
Molecular and cellular biology
35
1433-48
2015
Mostra il sommario
During spermiogenesis, the postmeiotic phase of mammalian spermatogenesis, transcription is progressively repressed as nuclei of haploid spermatids are compacted through a dramatic chromatin reorganization involving hyperacetylation and replacement of most histones with protamines. Although BRDT functions in transcription and histone removal in spermatids, it is unknown whether other BET family proteins play a role. Immunofluorescence of spermatogenic cells revealed BRD4 in a ring around the nuclei of spermatids containing hyperacetylated histones. The ring lies directly adjacent to the acroplaxome, the cytoskeletal base of the acrosome, previously linked to chromatin reorganization. The BRD4 ring does not form in acrosomal mutant mice. Chromatin immunoprecipitation followed by sequencing in spermatids revealed enrichment of BRD4 and acetylated histones at the promoters of active genes. BRD4 and BRDT show distinct and synergistic binding patterns, with a pronounced enrichment of BRD4 at spermatogenesis-specific genes. Direct association of BRD4 with acetylated H4 decreases in late spermatids as acetylated histones are removed from the condensing nucleus in a wave following the progressing acrosome. These data provide evidence of a prominent transcriptional role for BRD4 and suggest a possible removal mechanism for chromatin components from the genome via the progressing acrosome as transcription is repressed and chromatin is compacted during spermiogenesis. | Immunofluorescence | 25691659
 |
Epstein-Barr virus-mediated transformation of B cells induces global chromatin changes independent to the acquisition of proliferation.
Hernando, H; Islam, AB; Rodríguez-Ubreva, J; Forné, I; Ciudad, L; Imhof, A; Shannon-Lowe, C; Ballestar, E
Nucleic acids research
42
249-63
2014
Mostra il sommario
Epstein-Barr virus (EBV) infects and transforms human primary B cells inducing indefinite proliferation. To investigate the potential participation of chromatin mechanisms during the EBV-mediated transformation of resting B cells we performed an analysis of global changes in histone modifications. We observed a remarkable decrease and redistribution of heterochromatin marks including H4K20me3, H3K27me3 and H3K9me3. Loss of H4K20me3 and H3K9me3 occurred at constitutive heterochromatin repeats. For H3K27me3 and H3K9me3, comparison of ChIP-seq data revealed a decrease in these marks in thousands of genes, including clusters of HOX and ZNF genes, respectively. Moreover, DNase-seq data comparison between resting and EBV-transformed B cells revealed increased endonuclease accessibility in thousands of genomic sites. We observed that both loss of H3K27me3 and increased accessibility are associated with transcriptional activation. These changes only occurred in B cells transformed with EBV and not in those stimulated to proliferate with CD40L/IL-4, despite their similarities in the cell pathways involved and proliferation rates. In fact, B cells infected with EBNA-2 deficient EBV, which have much lower proliferation rates, displayed similar decreases for heterochromatic histone marks. Our study describes a novel phenomenon related to transformation of B cells, and highlights its independence of the pure acquisition of proliferation. | Western Blotting | 24097438
 |
Comparative analysis of genome-wide chromosomal histone modification patterns in maize cultivars and their wild relatives.
He, S; Yan, S; Wang, P; Zhu, W; Wang, X; Shen, Y; Shao, K; Xin, H; Li, S; Li, L
PloS one
9
e97364
2014
Mostra il sommario
Recent advances demonstrate that epigenome changes can also cause phenotypic diversity and can be heritable across generations, indicating that they may play an important role in evolutionary processes. In this study, we analyzed the chromosomal distribution of several histone modifications in five elite maize cultivars (B73, Mo17, Chang7-2, Zheng58, ZD958) and their two wild relatives (Zea mays L. ssp. parviglumis and Zea nicaraguensis) using a three-dimensional (3D) epigenome karyotyping approach by combining immunostaining and 3D reconstruction with deconvolution techniques. The distribution of these histone modifications along chromosomes demonstrated that the histone modification patterns are conserved at the chromosomal level and have not changed significantly following domestication. The comparison of histone modification patterns between metaphase chromosomes and interphase nuclei showed that some of the histone modifications were retained as the cell progressed from interphase into metaphase, although remodelling existed. This study will increase comprehension of the function of epigenetic modifications in the structure and evolution of the maize genome. | Immunocytochemistry | 24819606
 |
Altered nucleosome positioning at the transcription start site and deficient transcriptional initiation in Friedreich ataxia.
Chutake, YK; Costello, WN; Lam, C; Bidichandani, SI
The Journal of biological chemistry
289
15194-202
2014
Mostra il sommario
Most individuals with Friedreich ataxia (FRDA) are homozygous for an expanded GAA triplet repeat (GAA-TR) mutation in intron 1 of the FXN gene, which results in deficiency of FXN transcript. Consistent with the expanded GAA-TR sequence as a cause of variegated gene silencing, evidence for heterochromatin has been detected in intron 1 in the immediate vicinity of the expanded GAA-TR mutation in FRDA. Transcriptional deficiency in FRDA is thought to result from deficient elongation through the expanded GAA-TR sequence because of repeat-proximal heterochromatin and abnormal DNA structures adopted by the expanded repeat. There is also evidence for deficient transcriptional initiation in FRDA, but its relationship to the expanded GAA-TR mutation remains unclear. We show that repressive chromatin extends from the expanded GAA-TR in intron 1 to the upstream regions of the FXN gene, involving the FXN transcriptional start site. Using a chromatin accessibility assay and a high-resolution nucleosome occupancy assay, we found that the major FXN transcriptional start site, which is normally in a nucleosome-depleted region, is rendered inaccessible by altered nucleosome positioning in FRDA. Consistent with the altered epigenetic landscape the FXN gene promoter, a typical CpG island promoter, was found to be in a transcriptionally non-permissive state in FRDA. Both metabolic labeling of nascent transcripts and an unbiased whole transcriptome analysis revealed a severe deficiency of transcriptional initiation in FRDA. Deficient transcriptional initiation, and not elongation, is the major cause of FXN transcriptional deficiency in FRDA, and it is related to the spread of repressive chromatin from the expanded GAA-TR mutation. | | 24737321
 |
Establishment of regions of genomic activity during the Drosophila maternal to zygotic transition.
Li, XY; Harrison, MM; Villalta, JE; Kaplan, T; Eisen, MB
eLife
3
2014
Mostra il sommario
We describe the genome-wide distributions and temporal dynamics of nucleosomes and post-translational histone modifications throughout the maternal-to-zygotic transition in embryos of Drosophila melanogaster. At mitotic cycle 8, when few zygotic genes are being transcribed, embryonic chromatin is in a relatively simple state: there are few nucleosome free regions, undetectable levels of the histone methylation marks characteristic of mature chromatin, and low levels of histone acetylation at a relatively small number of loci. Histone acetylation increases by cycle 12, but it is not until cycle 14 that nucleosome free regions and domains of histone methylation become widespread. Early histone acetylation is strongly associated with regions that we have previously shown to be bound in early embryos by the maternally deposited transcription factor Zelda, suggesting that Zelda triggers a cascade of events, including the accumulation of specific histone modifications, that plays a role in the subsequent activation of these sequences. | | 25313869
 |
Histone methylation has dynamics distinct from those of histone acetylation in cell cycle reentry from quiescence.
Mews, P; Zee, BM; Liu, S; Donahue, G; Garcia, BA; Berger, SL
Molecular and cellular biology
34
3968-80
2014
Mostra il sommario
Cell growth is attuned to nutrient availability to sustain homeostatic biosynthetic processes. In unfavorable environments, cells enter a nonproliferative state termed quiescence but rapidly return to the cell cycle once conditions support energetic needs. Changing cellular metabolite pools are proposed to directly alter the epigenome via histone acetylation. Here we studied the relationship between histone modification dynamics and the dramatic transcriptional changes that occur during nutrient-induced cell cycle reentry from quiescence in the yeast Saccharomyces cerevisiae. SILAC (stable isotope labeling by amino acids in cell culture)-based mass spectrometry showed that histone methylation-in contrast to histone acetylation-is surprisingly static during quiescence exit. Chromatin immunoprecipitation followed by massive parallel sequencing (ChIP-seq) revealed genome-wide shifts in histone acetylation at growth and stress genes as cells exit quiescence and transcription dramatically changes. Strikingly, however, the patterns of histone methylation remain intact. We conclude that the functions of histone methylation and acetylation are remarkably distinct during quiescence exit: acetylation rapidly responds to metabolic state, while methylation is independent. Thus, the initial burst of growth gene reactivation emerging from quiescence involves dramatic increases of histone acetylation but not of histone methylation. | | 25154414
 |
Rpd3- and spt16-mediated nucleosome assembly and transcriptional regulation on yeast ribosomal DNA genes.
Johnson, JM; French, SL; Osheim, YN; Li, M; Hall, L; Beyer, AL; Smith, JS
Molecular and cellular biology
33
2748-59
2013
Mostra il sommario
Ribosomal DNA (rDNA) genes in eukaryotes are organized into multicopy tandem arrays and transcribed by RNA polymerase I. During cell proliferation, ∼50% of these genes are active and have a relatively open chromatin structure characterized by elevated accessibility to psoralen cross-linking. In Saccharomyces cerevisiae, transcription of rDNA genes becomes repressed and chromatin structure closes when cells enter the diauxic shift and growth dramatically slows. In this study, we found that nucleosomes are massively depleted from the active rDNA genes during log phase and reassembled during the diauxic shift, largely accounting for the differences in psoralen accessibility between active and inactive genes. The Rpd3L histone deacetylase complex was required for diauxic shift-induced H4 and H2B deposition onto rDNA genes, suggesting involvement in assembly or stabilization of the entire nucleosome. The Spt16 subunit of FACT, however, was specifically required for H2B deposition, suggesting specificity for the H2A/H2B dimer. Miller chromatin spreads were used for electron microscopic visualization of rDNA genes in an spt16 mutant, which was found to be deficient in the assembly of normal nucleosomes on inactive genes and the disruption of nucleosomes on active genes, consistent with an inability to fully reactivate polymerase I (Pol I) transcription when cells exit stationary phase. | | 23689130
 |
FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of αv and β3 integrin transcription.
Evellin, S; Galvagni, F; Zippo, A; Neri, F; Orlandini, M; Incarnato, D; Dettori, D; Neubauer, S; Kessler, H; Wagner, EF; Oliviero, S
Molecular and cellular biology
33
1198-209
2013
Mostra il sommario
To form three-dimensional capillary tubes, endothelial cells must establish contacts with the extracellular matrix that provides signals for their proliferation, migration, and differentiation. The transcription factor Fosl1 plays a key role in the vasculogenic and angiogenic processes as Fosl1 knockout embryos die with vascular defects in extraembryonic tissues. Here, we show that Fosl1(-/-) embryonic stem cells differentiate into endothelial cells but fail to correctly assemble into primitive capillaries and to form tube-like structures. FOSL1 silencing affects in vitro angiogenesis, increases cell adhesion, and decreases cell mobility of primary human endothelial cells (HUVEC). We further show that FOSL1 is a repressor of αv and β3 integrin expression and that the down-modulation of αvβ3 rescues the angiogenic phenotype in FOSL1-silenced HUVEC, while the ectopic expression of αvβ3 alone reproduces the phenotypic alterations induced by FOSL1 knockdown. FOSL1 represses the transcription of both αv and β3 integrin genes by binding together with JunD to their proximal promoter via the transcription factor SP1. These data suggest that FOSL1-dependent negative regulation of αvβ3 expression on endothelial cells is required for endothelial assembly into vessel structures. | Western Blotting, Immunofluorescence | 23319049
 |