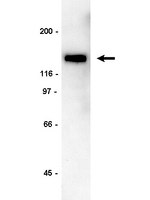
Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes.
Cortese, GP; Barrientos, RM; Maier, SF; Patterson, SL
The Journal of neuroscience : the official journal of the Society for Neuroscience
31
4274-9
2010
Mostra il sommario
For reasons that are not well understood, aging significantly increases brain vulnerability to challenging life events. High-functioning older individuals often experience significant cognitive decline after an inflammatory event such as surgery, infection, or injury. We have modeled this phenomenon in rodents and have previously reported that a peripheral immune challenge (intraperitoneal injection of live Escherichia coli) selectively disrupts consolidation of hippocampus-dependent memory in aged (24-month-old), but not young (3-month-old), F344xBN rats. More recently, we have demonstrated that this infection-evoked memory deficit is mirrored by a selective deficit in long-lasting synaptic plasticity in the hippocampus. Interestingly, these deficits occur in forms of long-term memory and synaptic plasticity known to be strongly dependent on brain-derived neurotrophic factor (BDNF). Here, we begin to test the hypothesis that the combination of aging and an infection might disrupt production or processing of BDNF protein in the hippocampus, decreasing the availability of BDNF for plasticity-related processes at synaptic sites. We find that mature BDNF is markedly reduced in Western blots of hippocampal synaptoneurosomes prepared from aged animals following infection. This reduction is blocked by intra-cisterna magna administration of the anti-inflammatory cytokine IL-1Ra (interleukin 1-specific receptor antagonist). Levels of the pan-neurotrophin receptor p75(NTR) and the BDNF receptor TrkB (tropomyosin receptor kinase B) are not significantly altered in these synaptoneurosomes, but phosphorylation of TrkB and downstream activation of PLCγ1 (phospholipase Cγ1) and ERK (extracellular response kinase) are attenuated-observations consistent with reduced availability of mature BDNF to activate TrkB signaling. These data suggest that inflammation-evoked reductions in BDNF at synapses might contribute to inflammation-evoked disruptions in long-term memory and synaptic plasticity in aging. | Western Blotting | 21411668
 |
The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function.
Dobenecker, MW; Schmedt, C; Okada, M; Tarakhovsky, A
Molecular and cellular biology
25
10533-42
2004
Mostra il sommario
Regulation of Src family kinase (SFK) activity is indispensable for a functional immune system and embryogenesis. The activity of SFKs is inhibited by the presence of the carboxy-terminal Src kinase (Csk) at the cell membrane. Thus, recruitment of cytosolic Csk to the membrane-associated SFKs is crucial for its regulatory function. Previous studies utilizing in vitro and transgenic models suggested that the Csk-binding protein (Cbp), also known as phosphoprotein associated with glycosphingolipid microdomains (PAG), is the membrane adaptor for Csk. However, loss-of-function genetic evidence to support this notion was lacking. Herein, we demonstrate that the targeted disruption of the cbp gene in mice has no effect on embryogenesis, thymic development, or T-cell functions in vivo. Moreover, recruitment of Csk to the specialized membrane compartment of "lipid rafts" is not impaired by Cbp deficiency. Our results indicate that Cbp is dispensable for the recruitment of Csk to the membrane and that another Csk adaptor, yet to be discovered, compensates for the loss of Cbp. Testo completo dell'articolo | Western Blotting | 16287865
 |
Phospholipase C and src tyrosine kinases mediate neurotensin-stimulated Cl- secretion in rabbit proximal colon.
Roli Prasad, Jayashree Venkatasubramanian, Milen Amde, Mrinalini Chatta Rao
Digestive diseases and sciences
49
1318-26
2004
Mostra il sommario
Calcium-dependent secretagogues, such as neurotensin, stimulate age-dependent chloride transport in rabbit distal colonocytes, but their action in the proximal colon is unknown. This study examines the effect of neurotensin on chloride transport and its mechanism of action in rabbit proximal colonocytes. Our results show that neurotensin stimulates chloride transport only in adult, and not weanling or newborn, colonocytes. The calcium ionophore A23187 shows similar age dependence, while PGE2, which acts via cAMP, stimulates transport in all ages. The roles of phospholipase C, tyrosine kinases, and src tyrosine kinases were examined using specific inhibitors, i.e., U73122, genistein, and PP2, respectively. All three agents significantly inhibit neurotensin-stimulated chloride transport in adult colonocytes. In conclusion, this study reports for the first time that neurotensin stimulates chloride secretion in rabbit proximal colonocytes. This is also the first demonstration that neurotensin action exhibits age dependence and is dependent on phospholipase C and src tyrosine kinase activity. | | 15387363
 |
Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc
Miranti, C. K., et al
J Biol Chem, 274:10571-81 (1999)
1998
| Immunoprecipitation, Immunoblotting (Western) | 10187852
 |
SH2-B is required for nerve growth factor-induced neuronal differentiation.
Rui, L, et al.
J. Biol. Chem., 274: 10590-4 (1999)
1998
Mostra il sommario
Nerve growth factor (NGF) is essential for the development and survival of sympathetic and sensory neurons. NGF binds to TrkA, activates the intrinsic kinase activity of TrkA, and promotes the differentiation of pheochromocytoma (PC12) cells into sympathetic-like neurons. Several signaling molecules and pathways are known to be activated by NGF, including phospholipase Cgamma, phosphatidylinositol-3 kinase, and the mitogen-activated protein kinase cascade. However, the mechanism of NGF-induced neuronal differentiation remains unclear. In this study, we examined whether SH2-Bbeta, a recently identified pleckstrin homology and SH2 domain-containing signaling protein, is a critical signaling protein for NGF. TrkA bound to glutathione S-transferase fusion proteins containing SH2-Bbeta, and NGF stimulation dramatically increased that binding. In contrast, NGF was unable to stimulate the association of TrkA with a glutathione S-transferase fusion protein containing a mutant SH2-Bbeta(R555E) with a defective SH2 domain. When overexpressed in PC12 cells, SH2-Bbeta co-immunoprecipitated with TrkA in response to NGF. NGF stimulated tyrosyl phosphorylation of endogenous SH2-Bbeta as well as exogenously expressed GFP-SH2-Bbeta but not GFP-SH2-Bbeta(R555E). Overexpression of SH2-Bbeta(R555E) blocked NGF-induced neurite outgrowth of PC12 cells, whereas overexpression of wild type SH2-Bbeta enhanced NGF-induced neurite outgrowth. Overexpression of either wild type or mutant SH2-Bbeta(R555E) did not alter tyrosyl phosphorylation of TrkA, Shc, or phospholipase Cgamma in response to NGF or NGF-induced activation of ERK1/2, suggesting that SH2-Bbeta may initiate a previously unknown pathway(s) that is essential for NGF-induced neurite outgrowth. Taken together, these data indicate that SH2-Bbeta is a novel signaling molecule required for NGF-induced neuronal differentiation. | Immunoblotting (Western) | 10187854
 |
Tyrosine phosphorylation and recruitment of ZAP-70 to the CD3-TCR complex are defective after CD2 stimulation
Hubert, P., et al
J Immunol, 157:4322-32 (1996)
1996
| Immunoprecipitation | 8906806
 |
T cell activation-dependent association between the p85 subunit of the phosphatidylinositol 3-kinase and Grb2/phospholipase C-gamma 1-binding phosphotyrosyl protein pp36/38.
Fukazawa, T, et al.
J. Biol. Chem., 270: 20177-82 (1995)
1994
Mostra il sommario
Tyrosine phosphorylation of cellular proteins is an early and an essential step in T cell receptor-mediated lymphocyte activation. Tyrosine phosphorylation of transmembrane receptor chains (such as zeta and CD3 chains) and membrane-associated proteins provides docking sites for SH2 domains of adaptor proteins and signaling enzymes, resulting in their recruitment in the vicinity of activated receptors. pp36/38 is a prominent substrate of early tyrosine phosphorylation upon stimulation through the T cell receptor. The tyrosine-phosphorylated form of pp36/38 is membrane-associated and directly interacts with phospholipase C-gamma 1 and Grb2, providing one mechanism to recruit downstream effectors to the cell membrane. Here, we demonstrate that in Jurkat T cells, pp36/38 associates with the p85 subunit of phosphatidylinositol 3-kinase (PI-3-K p85) in an activation-dependent manner. Association of pp36/38 with PI-3-K p85 was confirmed by transfection of a hemagglutinin-tagged p85 alpha cDNA into Jurkat cells followed by anti-hemagglutinin immunoprecipitation. In vitro binding experiments with glutathione S-transferase fusion proteins of PI-3-K p85 demonstrated that the SH2 domains, but not the SH3 domain, mediated binding to pp36/38. This binding was selectively abrogated by phosphopeptides that bind to p85 SH2 domains with high affinity. Filter binding assays demonstrated that association between pp36/38 and PI-3-K p85 SH2 domains was due to direct binding. These results strongly suggest the role of pp36/38 in recruiting PI-3-K to the cell membrane and further support the idea that pp36/38 is a multifunctional docking protein for SH2 domain-containing signaling proteins in T cells. | Immunoprecipitation, Immunoblotting (Western) | 7544353
 |
Phosphorylation of Nck in response to a variety of receptors, phorbol myristate acetate, and cyclic AMP.
Park, D and Rhee, S G
Mol. Cell. Biol., 12: 5816-23 (1992)
1992
Mostra il sommario
The 47-kDa protein coimmunoprecipitated with phospholipase C (PLC)-gamma 1 by anti-PLC-gamma 1 monoclonal antibodies is proved to be Nck, a protein composed almost exclusively of one SH2 and three SH3 domains. Nck and PLC-gamma 1 are recognized by certain anti-PLC-gamma 1 monoclonal antibodies because Nck and PLC-gamma 1 share an epitope that likely is located in their SH3 domains. Nck is widely distributed in rat tissues, with an especially high level of expression in testes. The expression levels of Nck remains unchanged during the development of rat brain, whereas PLC-gamma 1 decreases during the same developmental period. Stimulation of A431 cells with epidermal growth factor elicits the tight association of Nck with the epidermal growth factor receptor and phosphorylation of Nck on both serine and tyrosine residues. The phosphorylation of Nck is also enhanced in response to stimulation of the nerve growth factor receptor in PC12 cells, the T-cell receptor complex in Jurkat cells, the membrane immunoglobulin M in Daudi cells, and the low-affinity immunoglobulin G receptor (Fc gamma RII) in U937 cells. The phosphorylation of Nck was also enhanced following treatment of A431 cells with phorbol 12-myristate 13-acetate or forskolin. These results suggest that Nck is a target for a variety of protein kinases that might modulate the postulated role of Nck as an adaptor for the physical and functional coordination of signalling proteins. | | 1333046
 |
CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line.
Park, D J, et al.
Proc. Natl. Acad. Sci. U.S.A., 88: 5453-6 (1991)
1991
Mostra il sommario
The human T-cell line Jurkat was found to contain at least two immunologically distinct isoforms of inositol phospholipid-specific phospholipase C (PLC), PLC-beta 1 and PLC-gamma 1. Treatment of Jurkat cells with antibody to CD3 led to phosphorylation of PLC-gamma 1 but not of PLC-beta 1. The phosphorylation of PLC-gamma 1 occurred rapidly and transiently on both serine and tyrosine residues; tyrosine phosphorylation reached a maximum level less than 1 min after stimulation and decreased rapidly, both in the presence and in the absence of orthovanadate. Two-dimensional phosphopeptide map analysis revealed that the major sites of tyrosine and serine phosphorylation in PLC-gamma 1 from activated Jurkat cells are the same as those in PLC-gamma 1 from cells treated with peptide growth factors such as epidermal growth factor and platelet-derived growth factor. Previously, it has been shown that multiple phosphorylation of PLC-gamma 1 by the growth factor receptor tyrosine kinases leads to activation of PLC-gamma 1. Thus, the current data suggest that inositol phospholipid hydrolysis triggered by the T-cell antigen receptor-CD3 complex is due, at least in part, to activation of PLC-gamma 1 and that the mechanism by which this activation is achieved involves phosphorylation of multiple tyrosine residues on PLC-gamma 1 by a nonreceptor tyrosine kinase coupled to the T-cell antigen receptor-CD3 complex. | | 1828897
 |
Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro.
Meisenhelder, J, et al.
Cell, 57: 1109-22 (1989)
1988
Mostra il sommario
Phospholipase C-gamma (PLC-gamma) was rapidly phosphorylated on tyrosines and serines following PDGF and EGF treatment of quiescent 3T3 mouse fibroblasts and A431 human epidermoid cells, respectively, PDGF treatment increased PLC-gamma phosphorylation within 30 sec. This lasted for up to 1 hr, and occurred at high stoichiometry. Continuous receptor occupancy was required to maintain this phosphorylation. Three major sites of tyrosine phosphorylation were detected in PLC-gamma, two of which were phosphorylated in EGF-treated A431 cells. Under certain conditions PDGF receptor coimmunoprecipitated with PLC-gamma, suggesting that PDGF receptor can phosphorylate PLC-gamma directly. Indeed, purified PDGF or EGF receptor phosphorylated purified PLC-gamma on tyrosines identical to those phosphorylated in vivo. Tyrosine phosphorylation of PLC-gamma was not induced by bombesin, TPA, or insulin. Stimulation of PLC-gamma tyrosine phosphorylation and the reported ability of PDGF and EGF to induce phosphatidylinositol turnover in different cells were strongly correlated. We propose that tyrosine phosphorylation of PLC-gamma by PDGF and EGF receptors leads to its activation, and a consequent increase in phosphatidylinositol turnover. | | 2472219
 |