Anterograde trafficking of neurotrophin-3 in the adult olfactory system in vivo.
Liu, H; Lu, M; Guthrie, KM
Experimental neurology
241
125-37
2013
Mostra il sommario
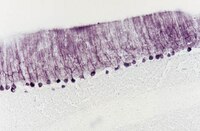
The olfactory system continuously incorporates new neurons into functional circuits throughout life. Axons from olfactory sensory neurons (OSNs) in the nasal cavity synapse on mitral, tufted and periglomerular (PG) cells in the main olfactory bulb, and low levels of turnover within the OSN population results in ingrowth of new axons under normal physiological conditions. Subpopulations of bulb interneurons are continually eliminated by apoptosis, and are replaced by new neurons derived from progenitors in the adult forebrain subventricular zone. Integration of new neurons, including PG cells that are contacted by sensory axons, leads to ongoing reorganization of adult olfactory bulb circuits. The mechanisms regulating this adaptive structural plasticity are not all known, but the process is reminiscent of early nervous system development. Neurotrophic factors have well-established roles in controlling neuronal survival and connectivity during development, leading to speculation that trophic interactions between OSNs and their target bulb neurons may mediate some of these same processes in adults. A number of different trophic factors and their cognate receptors are expressed in the adult olfactory pathway. Neurotrophin-3 (NT3) is among these, as reflected by beta-galactosidase expression in transgenic reporter mice expressing lacZ under the NT3 promoter. Using a combination of approaches, including immunocytochemistry, real-time PCR of laser-captured RNA, and adenovirus-mediated gene transfer of NT3 fusion peptides in vivo, we demonstrate that OSNs express and anterogradely transport NT3 to the olfactory bulb. We additionally observe that in mice treated with adenovirus encoding NT3 tagged with hemagglutinin (HA), a subset of bulb neurons expressing the TrkC neurotrophin receptor are immunoreactive for HA, suggesting their acquisition of the fusion peptide from infected sensory neurons. Our results therefore provide evidence that OSNs may serve as an afferent source of trophic signals for the adult mouse olfactory bulb. | 23261763
 |
Peripheral projections of rat primary sensory neurons immunoreactive for neurotrophin 3.
Zhou, X F and Rush, R A
J. Comp. Neurol., 363: 69-77 (1995)
1994
Mostra il sommario
Sensory neurons can be classified into subpopulations based on a variety of characteristics, including their morphology and physiological modalities. Whether any of these classifications correlates with neurotrophic sensitivities has not been determined. We have recently reported that a subpopulation of large diameter sensory neurons of the rat contain neurotrophin 3-like immunoreactivity (NT3-ir). In this study, we have further characterised NT3-ir sensory neurons by their size, segmental localization, and peripheral projections by combined techniques of retrograde tracing and immunohistochemistry. The size distribution showed that NT3-ir was localised to a subpopulation of large-diameter neurons ranging from 560 to 3,120 microns2. Greater numbers of NT3-ir neurons reside in trigeminal (43% of total), cervical (36%), and lumbar (39%) than in thoracic spinal ganglia (13-17%). In combination with Fluoro-Gold retrograde tracing, it was found that about 30% of sensory neurons projecting to the tibial muscle were NT3-ir, compared with 39% for tendon, 50% for whisker hair follicles, 17% for subdermis or epidermis, and only 1% for kidney or adrenal gland. These studies indicate that NT3-ir sensory neurons mainly project to skin and muscles but not viscera. Thus, the characterization of NT3-ir spinal sensory neurons suggests that large sensory neurons subserving proprioception and mechanoception require NT3 for the maintenance of normal function. | 8682938
 |
Localization of neurotrophin-3-like immunoreactivity in the rat central nervous system.
Zhou, X F and Rush, R A
Brain Res., 643: 162-72 (1994)
1993
Mostra il sommario
Neurotropin-3 (NT3) is a nerve growth factor (NGF) homologue whose function is presently unknown. The factor promotes the survival of a subpopulation of sensory and sympathetic neurons in vitro. NT3 mRNA is widely distributed in both the peripheral and central nervous system but the distribution of NT3 has not yet been examined. In the present study we have determined the regional distribution and cellular localization of NT3-like immunoreactivity (-IR) in the central nervous system by immunohistochemistry. Both glia and neurons were stained. NT3-IR glia were distributed in corpus callosum, substantia nigra, fimbria of hippocampus, subependymal areas of the ventricles and cerebellum. In the forebrain, NT3-IR was detected in a number of neuronal cells, including pyramidal cells in the fifth layer of the cerebral cortices, subpopulations of neurons in the septal nuclei, diagonal bands of Broca, olfactory primary cortex, amygdala and islands of Calleja. In the hippocampus, pyramidal cells in the CA1, CA2 and lateral regions of CA3 and granular cells in dorsal dentate gyrus were labelled with different intensities. Neurons in the bed nuclei of the striatum terminalis, mesencephalic trigeminal nuclei and motoneurons in the brain stem and spinal cord were intensively labelled. A subpopulation of neurons in the reticular thalamic nuclei and midbrain were moderately labelled. Finally, in the cerebellum, NT3-IR was also found in Purkinje cells and neurons in the deep cerebellar nuclei. In some brain regions such as hippocampus, the distribution of NT3-IR correlates with that of mRNANT3 as described by others. In contrast in other regions such as spinal cord and brain stem, little correlation was found between protein and mRNA. The results suggest that some NT3 immunoreactive neurons in the central nervous system accumulate NT3 in accord with a neurotrophic role for their maintenance or survival, while others may synthesize and secrete the factor to provide support for innervating neurons. | 8032912
 |