Regulatory mechanisms of testosterone-stimulated song in the sensorimotor nucleus HVC of female songbirds.
Dittrich, F; Ramenda, C; Grillitsch, D; Frankl-Vilches, C; Ko, MC; Hertel, M; Goymann, W; ter Maat, A; Gahr, M
BMC neuroscience
15
128
2014
Mostra il sommario
In male birds, influence of the sex steroid hormone testosterone and its estrogenic metabolites on seasonal song behavior has been demonstrated for many species. In contrast, female song was only recently recognized to be widespread among songbird species, and to date, sex hormone effects on singing and brain regions controlling song development and production (song control nuclei) have been studied in females almost exclusively using domesticated canaries (Serinus canaria). However, domesticated female canaries hardly sing at all in normal circumstances and exhibit only very weak, if any, song seasonally under the natural photoperiod. By contrast, adult female European robins (Erithacus rubecula) routinely sing during the winter season, a time when they defend feeding territories and show elevated circulating testosterone levels. We therefore used wild female European robins captured in the fall to examine the effects of testosterone administration on song as well as on the anatomy and the transcriptome of the song control nucleus HVC (sic). The results obtained from female robins were compared to outcomes of a similar experiment done in female domesticated canaries.Testosterone treatment induced abundant song in female robins. Examination of HVC transcriptomes and histological analyses of song control nuclei showed testosterone-induced differentiation processes related to neuron growth and spacing, angiogenesis and neuron projection morphogenesis. Similar effects were found in female canaries treated with testosterone. In contrast, the expression of genes related to synaptic transmission was not enhanced in the HVC of testosterone treated female robins but was strongly up-regulated in female canaries. A comparison of the testosterone-stimulated transcriptomes indicated that brain-derived neurotrophic factor (BDNF) likely functions as a common mediator of the testosterone effects in HVC.Testosterone-induced singing of female robins correlated with cellular differentiation processes in the HVC that were partially similar to those seen in the HVC of testosterone-treated female canaries. Other modes of testosterone action, notably related to synaptic transmission, appeared to be regulated in a more species-specific manner in the female HVC. Divergent effects of testosterone on the HVC of different species might be related to differences between species in regulatory mechanisms of the singing behavior. | | 25442096
 |
Ablation of proliferating cells in the CNS exacerbates motor neuron disease caused by mutant superoxide dismutase.
Audet, JN; Gowing, G; Paradis, R; Soucy, G; Julien, JP
PloS one
7
e34932
2011
Mostra il sommario
Proliferation of glia and immune cells is a common pathological feature of many neurodegenerative diseases including amyotrophic lateral sclerosis (ALS). Here, to investigate the role of proliferating cells in motor neuron disease, SOD1(G93A) transgenic mice were treated intracerebroventicularly (i.c.v.) with the anti-mitotic drug cytosine arabinoside (Ara-C). I.c.v. delivery of Ara-C accelerated disease progression in SOD1(G93A) mouse model of ALS. Ara-C treatment caused substantial decreases in the number of microglia, NG2+ progenitors, Olig2+ cells and CD3+ T cells in the lumbar spinal cord of symptomatic SOD1(G93A) transgenic mice. Exacerbation of disease was also associated with significant alterations in the expression inflammatory molecules IL-1β, IL-6, TGF-β and the growth factor IGF-1. | | 22523565
 |
Decreased expression of neuronal nitric oxide synthase in the nucleus tractus solitarii inhibits sympathetically mediated baroreflex responses in rat.
Lin, LH; Nitschke Dragon, D; Jin, J; Tian, X; Chu, Y; Sigmund, C; Talman, WT
The Journal of physiology
590
3545-59
2011
Mostra il sommario
Despite numerous studies it remains controversial whether nitric oxide (NO·) synthesized by neuronal NOS (nNOS) plays an excitatory or inhibitory role in transmission of baroreflex signals in the nucleus tractus solitarii (NTS). In the current studies we sought to test the hypothesis that nNOS is involved in excitation of baroreflex pathways in NTS while excluding pharmacological interventions in assessing the influence of nNOS. We therefore developed, validated and utilized a short hairpin RNA (shRNA) to reduce expression of nNOS in the NTS of rats whose baroreflex activity was then studied. We demonstrate downregulation of nNOS through transduction with adeno-associated virus type 2 (AAV2) carrying shRNA for nNOS. When injected bilaterally into NTS AAV2nNOSshRNA significantly reduced reflex tachycardic responses to acute hypotension while not affecting reflex bradycardic responses to acute increases of arterial pressure. Control animals treated with intravenous propranolol to block sympathetically mediated chronotropic responses manifested the same baroreflex responses as animals that had been treated with AAV2nNOSshRNA. Neither AAV2 eGFP nor AAV2nNOScDNA affected baroreflex responses. Blocking cardiac vagal influences with atropine similarly reduced baroreflex-mediated bradycardic responses to increases in arterial pressure both in control animals and in those treated with AAV2nNOSshRNA. We conclude that NO· synthesized by nNOS in the NTS is integral to excitation of baroreflex pathways involved in reflex tachycardia, a largely sympathetically mediated response, but not reflex bradycardia, a largely parasympathetically mediated response. We suggest that, at the basal state, nNOS is maximally engaged. Thus, its upregulation does not augment the baroreflex. | | 22687614
 |
Sensory-motor deficits and neurofilament disorganization in gigaxonin-null mice.
Ganay, T; Boizot, A; Burrer, R; Chauvin, JP; Bomont, P
Molecular neurodegeneration
6
25
2010
Mostra il sommario
Giant Axonal Neuropathy (GAN) is a fatal neurodegenerative disorder with early onset characterized by a severe deterioration of the peripheral and central nervous system, involving both the motor and the sensory tracts and leading to ataxia, speech defect and intellectual disabilities. The broad deterioration of the nervous system is accompanied by a generalized disorganization of the intermediate filaments, including neurofilaments in neurons, but the implication of this defect in disease onset or progression remains unknown. The identification of gigaxonin, the substrate adaptor of an E3 ubiquitin ligase, as the defective protein in GAN allows us to now investigate the crucial role of the gigaxonin-E3 ligase in sustaining neuronal and intermediate filament integrity. To study the mechanisms controlled by gigaxonin in these processes and to provide a relevant model to test the therapeutic approaches under development for GAN, we generated a Gigaxonin-null mouse by gene targeting.We investigated for the first time in Gigaxonin-null mice the deterioration of the motor and sensory functions over time as well as the spatial disorganization of neurofilaments. We showed that gigaxonin depletion in mice induces mild but persistent motor deficits starting at 60 weeks of age in the 129/SvJ-genetic background, while sensory deficits were demonstrated in C57BL/6 animals. In our hands, another gigaxonin-null mouse did not display the early and severe motor deficits reported previously. No apparent neurodegeneration was observed in our knock-out mice, but dysregulation of neurofilaments in proximal and distal axons was massive. Indeed, neurofilaments were not only more abundant but they also showed the abnormal increase in diameter and misorientation that are characteristics of the human pathology.Together, our results show that gigaxonin depletion in mice induces mild motor and sensory deficits but recapitulates the severe neurofilament dysregulation seen in patients. Our model will allow investigation of the role of the gigaxonin-E3 ligase in organizing neurofilaments and may prove useful in understanding the pathological processes engaged in other neurodegenerative disorders characterized by accumulation of neurofilaments and dysfunction of the Ubiquitin Proteasome System, such as Amyotrophic Lateral Sclerosis, Huntington's, Alzheimer's and Parkinson's diseases. Testo completo dell'articolo | | 21486449
 |
The BTB and CNC homology 1 (BACH1) target genes are involved in the oxidative stress response and in the control of the cell cycle
Warnatz HJ, Schmidt D, Manke T, Piccini I, Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M, Lehrach H, Yaspo ML
J Biol Chem
2010
Mostra il sommario
The regulation of gene expression in response to environmental signals and metabolic imbalances is a key step in maintaining cellular homeostasis. BTB and CNC homology 1 (BACH1) is a heme-binding transcription factor repressing the transcription from a subset of MAF recognition elements (MAREs) at low intracellular heme levels. Upon heme binding, BACH1 is released from the MAREs, resulting in increased expression of antioxidant response genes. To systematically address the gene regulatory networks involving BACH1, we combined chromatin immunoprecipitation-sequencing (ChIP-seq) analysis of BACH1 target genes in HEK 293 cells with knock-down of BACH1 using three independent types of small interfering RNAs followed by transcriptome profiling using microarrays. The 59 BACH1 target genes identified by ChIP-seq were found highly enriched in genes showing expression changes after BACH1 knock-down, demonstrating the impact of BACH1 repression on transcription. In addition to known and new BACH1 targets involved in heme degradation (HMOX1, FTL, FTH1, ME1, SLC48A1) and redox regulation (GCLC, GCLM, SLC7A11), we also discovered BACH1 target genes effecting cell cycle and apoptosis pathways (ITPR2, CALM1, SQSTM1, TFE3, EWSR1, CDK6, BCL2L11, MAFG) as well as subcellular transport processes (CLSTN1, PSAP, MAPT, vault RNA). The newly identified impact of BACH1 on genes involved in neurodegenerative processes and proliferation provides an interesting basis for future dissection of BACH1-mediated gene repression in neurodegeneration and virus-induced cancerogenesis. | | 21555518
 |
Extended secondhand tobacco smoke exposure induces plasticity in nucleus tractus solitarius second-order lung afferent neurons in young guinea pigs.
Shin-Ichi Sekizawa,Chao-Yin Chen,Andrea G Bechtold,Jocelyn M Tabor,John M Bric,Kent E Pinkerton,Jesse P Joad,Ann C Bonham
The European journal of neuroscience
28
2008
Mostra il sommario
Infants and young children experiencing extended exposure to secondhand smoke (SHS) have an increased occurrence of asthma, as well as increased cough, wheeze, mucus production and airway hyper-reactivity. Plasticity in lung reflex pathways has been implicated in causing these symptoms, as have changes in substance P-related mechanisms. Using whole-cell voltage-clamp recordings and immunohistochemistry in brainstem slices containing anatomically identified second-order lung afferent nucleus tractus solitarius (NTS) neurons, we determined whether extended SHS exposure during the equivalent period of human childhood modified evoked or spontaneous excitatory synaptic transmission, and whether those modifications were altered by endogenous substance P. SHS exposure enhanced evoked synaptic transmission between sensory afferents and the NTS second-order neurons by eliminating synaptic depression of evoked excitatory postsynaptic currents (eEPSCs), an effect reversed by the neurokinin-1-receptor antagonist (SR140333). The recruitment of substance P in enhancing evoked synaptic transmission was further supported by an increased number of substance P-expressing lung afferent central terminals synapsing onto the second-order lung afferent neurons. SHS exposure did not change background spontaneous EPSCs. The data suggest that substance P in the NTS augments evoked synaptic transmission of lung sensory input following extended exposure to a pollutant. The mechanism may help to explain some of the exaggerated respiratory responses of children exposed to SHS. | | 18657181
 |
Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase.
Gowing, G; Philips, T; Van Wijmeersch, B; Audet, JN; Dewil, M; Van Den Bosch, L; Billiau, AD; Robberecht, W; Julien, JP
The Journal of neuroscience : the official journal of the Society for Neuroscience
28
10234-44
2008
Mostra il sommario
Microglial activation is a hallmark of all neurodegenerative diseases including amyotrophic lateral sclerosis (ALS). Here, a detailed characterization of the microglial cell population within the spinal cord of a mouse model of familial ALS was performed. Using flow cytometry, we detected three distinct microglial populations within the spinal cord of mice overexpressing mutant superoxide dismutase (SOD1): mature microglial cells (CD11b(+), CD45(low)), myeloid precursor cells (CD11b(+), CD45(int)), and macrophages (CD11b(+), CD45(high)). Characterization of cell proliferation within the CNS of SOD1(G93A) mice revealed that the expansion in microglial cell population is mainly attributable to the proliferation of myeloid precursor cells. To assess the contribution of proliferating microglia in motor neuron degeneration, we generated CD11b-TK(mut-30); SOD1(G93A) doubly transgenic mice that allow the elimination of proliferating microglia on administration of ganciclovir. Surprisingly, a 50% reduction in reactive microglia specifically in the lumbar spinal cord of CD11b-TK(mut-30); SOD1(G93A) doubly transgenic mice had no effect on motor neuron degeneration. This suggests that proliferating microglia-expressing mutant SOD1 are not central contributors of the neurodegenerative process in ALS caused by mutant SOD1. | | 18842883
 |
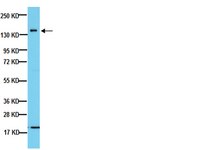
Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments.
Millecamps, S; Gowing, G; Corti, O; Mallet, J; Julien, JP
The Journal of neuroscience : the official journal of the Society for Neuroscience
27
4947-56
2007
Mostra il sommario
We generated mice with doxycycline control of a human neurofilament light (NF-L) transgene in the context of the absence (tTA;hNF-L;NF-L(-/-)) or presence (tTA;hNF-L;NF-L(+/-)) of endogenous mouse NF-L proteins. Doxycycline treatment caused the rapid disappearance of human NF-L (hNF-L) mRNA in tTA;hNF-L mice, but the hNF-L proteins remained with a half-life of 3 weeks in the brain. In the sciatic nerve, the disappearance of hNF-L proteins after doxycycline treatment occurred in synchrony along the sciatic nerve, suggesting a proteolysis of NF proteins along the entire axon. The presence of permanent NF network in tTA;hNF-L;NF-L(+/-) mice further stabilized and extended longevity of hNF-L proteins by several months. Surprisingly, after cessation of doxycycline treatment, there was no evidence of leading front of newly synthesized hNF-L proteins migrating into sciatic nerve axons devoid of NF structures. The hNF-L proteins detected at weekly intervals reappeared and accumulated in synchrony at similar rate along nerve segments, a phenomenon consistent with a fast hNF-L transport into axons. We estimated the hNF-L transport rate to be of approximately 10 mm/d in axons devoid of NF structures based on the use of an adenovirus encoding tet-responsive transcriptional activator to transactivate the hNF-L transgene in hypoglossal motor neurons. These results provide in vivo evidence that the stationary NF network in axons is a key determinant of half-life and transport rate of NF proteins. | Western Blotting | 17475803
 |
Postganglionic nerve stimulation induces temporal inhibition of excitability in rabbit sinoatrial node.
Fedorov, VV; Hucker, WJ; Dobrzynski, H; Rosenshtraukh, LV; Efimov, IR
American journal of physiology. Heart and circulatory physiology
291
H612-23
2005
Mostra il sommario
Vagal stimulation results in complex changes of pacemaker excitability in the sinoatrial node (SAN). To investigate the vagal effects in the rabbit SAN, we used optical mapping, which is the only technology that allows resolving simultaneous changes in the activation pattern and action potentials morphologies. With the use of immunolabeling, we identified the SAN as a neurofilament 160-positive but connexin 43-negative region (n = 5). Normal excitation originated in the SAN center with a cycle length (CL) of 405 +/- 14 ms (n = 14), spread anisotropically along the crista terminalis (CT), and failed to conduct toward the septum. Postganglionic nerve stimulation (PNS, 400-800 ms) reduced CL by 74 +/- 7% transiently and shifted the leading pacemaker inferiorly (78%) or superiorly (22%) from the SAN center by 2-10 mm. In the intercaval region between the SAN center and the septal block zone, PNS produced an 8 +/- 1-mm(2) region of transient hyperpolarization and inexcitability. The first spontaneous or paced excitation following PNS could not enter this region for 500-1,500 ms. Immunolabeling revealed that the PNS-induced inexcitable region is located between the SAN center and the block zone and has a 2.5-fold higher density of choline acetyltransferase than CT but is threefold lower than the SAN center. The fact that the inexcitability region does not coincide with the most innervated area indicates that the properties of the myocytes themselves, as well as intercellular coupling, must play a role in the inexcitability induction. Optically mapping revealed that PNS resulted in transient loss of pacemaker cell excitability and unidirectional entrance conduction block in the periphery of SAN. | | 16565321
 |
The immunological relatedness of neurofilament proteins of higher vertebrates.
Shaw, G, et al.
Eur. J. Cell Biol., 34: 130-6 (1984)
1983
Mostra il sommario
We prepared intermediate filaments from the nervous system of several different species, representing mammals, birds and reptiles. These were examined using a panel of polyclonal and monoclonal antibodies originally raised against pig or rat neurofilament proteins. All species studied possessed a single major protein of apparent molecular weight between 68 K and 75 K immunologically related to the lowest molecular weight rat and pig neurofilament protein. All birds and mammals possessed two proteins immunologically related respectively to the pig and rat middle and high molecular weight neurofilament proteins. These data show that the neurofilament triplet proteins represent an evolutionarily conserved three member protein family in birds and mammals, and allow us to suggest a new nomenclature for these three homologous proteins: "H" for the heaviest subunit, "M" for the middle subunit and "L" for the lightest subunit. We found that many monoclonal antibodies stained both the H- and M-proteins of all mammalian and avian species examined, suggesting a close immunological relatedness between these two proteins. The reptiles examined appeared to have only one high molecular weight protein, which was immunologically related to both of the high molecular weight mammalian and avian neurofilament proteins. We also noted a curious situation in neurofilament preparations derived from cows. Both the highest and the middle cow neurofilament proteins were stained by all antibodies which were specific solely for the high molecular weight protein in other species. | | 6203748
 |