Ultraviolet radiation inhibits interleukin-2-induced tyrosine phosphorylation and the activation of STAT5 in T lymphocytes.
Kulms, D; Schwarz, T
The Journal of biological chemistry
276
12849-55
2001
Mostra il sommario
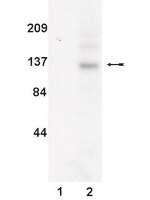
UV radiation was recently found to hinder interferon-gamma from exerting its biological effects by inhibiting the phosphorylation of signal transducer and activator of transcription (STAT)-1, a crucial signal transducing protein in the interferon-gamma pathway. Because this activity by UV may contribute to its immunosuppressive properties we studied whether this is specific for STAT1 or whether UV also affects other members of the STAT family. STAT5 is crucially involved in signaling of interleukin (IL)-2, enabling up-regulation of the IL-2 receptor alpha chain, an essential component of the high affinity IL-2 receptor. Exposure of the murine T cell line CTLL to IL-2 caused tyrosine phosphorylation of STAT5 that was remarkably reduced when cells were exposed to UV. Accordingly, STAT5 binding activity was significantly impaired in UV-exposed cells. In contrast, IL-2-induced tyrosine phosphorylation of the kinases Jak1 and Jak3 located upstream of STAT5 was not affected by UV. The effect of UV on STAT5 phosphorylation was antagonized by orthovanadate, implying involvement of a phosphatase in this process. Accordingly, up-regulation of the IL-2 receptor alpha chain was reduced in cells that were treated with IL-2 plus UV. Because STAT5-mediated IL-2 effects are vital for normal immune functions, inhibition of STAT5 signaling by UV may contribute to its well known immunosuppressive properties. | | 11278301
 |
Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2.
Johnston, J A, et al.
Nature, 370: 151-3 (1994)
1993
Mostra il sommario
Interleukin-2 is an autocrine growth factor for T cells which also activates other cells including B cells and natural killer cells. The subunits of the interleukin-2 receptor (IL-2R) lack intrinsic enzymatic activity, but protein tyrosine phosphorylation is a critical event following ligand binding and src family kinases, such as Lck, are known to be activated by IL-2 (refs 5-9). However, IL-2 signalling can occur in the absence of receptor interaction with Lck, suggesting that other protein tyrosine kinases might be important. Here we report that a new member of the Janus family of kinases (Jak-3) is coupled to the IL-2R in human peripheral blood T cells and natural killer cells. | | 8022485
 |
Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells.
Witthuhn, B A, et al.
Nature, 370: 153-7 (1994)
1993
Mostra il sommario
Many cytokines function through interaction with receptors of the cytokine receptor superfamily. Although lacking catalytic domains, cytokine receptors couple ligand binding to induction of protein tyrosine phosphorylation. Recent studies have shown that one or more of the Janus kinase family members (Jaks) associate with cytokine receptors and are tyrosine phosphorylated and activated following ligand binding. Here we describe a new Jak family kinase, Jak-3, and demonstrate that Jak-3, and to a lesser extent Jak-1, are tyrosine phosphorylated and Jak-3 is activated in the responses to interleukin-2 and interleukin-4 in T cells and myeloid cells. Jak-3 activation requires the serine-rich, membrane-proximal domain of the interleukin-2 receptor beta-chain, but does not require the acidic domain that is required for association and activation of Src family kinases. | | 8022486
 |
Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes.
Kawamura, M, et al.
Proc. Natl. Acad. Sci. U.S.A., 91: 6374-8 (1994)
1993
Mostra il sommario
Protein-tyrosine kinases (PTKs) are critical enzymes for receptor-mediated signaling in lymphocytes. Because natural killer (NK) cells are large granular lymphocytes with specialized effector function, we set out to identify PTKs preferentially expressed in these cells. One such PTK was identified and molecularly cloned. The predicted amino acid sequence shows that this kinase lacks SH2 or SH3 domains typical of src family kinases but has tandem nonidentical catalytic domains, indicating that it is a member of the Janus family of PTKs. Immunoprecipitation using antiserum generated against a peptide corresponding to the deduced amino acid sequence of this gene revealed a kinase with a molecular weight of approximately 125,000. The pattern of expression of this kinase contrasted sharply with that of other Janus kinases, which are ubiquitously expressed. The kinase described in the present study was found to be more limited in its expression; expression was found in NK cells and an NK-like cell line but not in resting T cells or in other tissues. In contrast, stimulated and transformed T cells expressed the gene, suggesting a role in lymphoid activation. Because of its homology and tissue expression, we have tentatively termed this PTK gene L-JAK for leukocyte Janus kinase. | | 8022790
 |