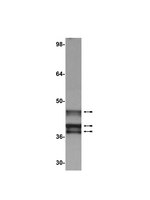
The Cellular Protein Complex Associated with a Transforming Region of E1A Contains c-MYC.
Vijayalingam, S; Subramanian, T; Zhao, LJ; Chinnadurai, G
Journal of virology
1070-9
2015
Mostra il sommario
The cell-transforming activity of human adenovirus 5 (hAd5) E1A is mediated by the N-terminal half of E1A, which interacts with three different major cellular protein complexes, p300/CBP, TRRAP/p400, and pRb family members. Among these protein interactions, the interaction of pRb family proteins with conserved region 2 (CR2) of E1A is known to promote cell proliferation by deregulating the activities of E2F family transcription factors. The functional consequences of interaction with the other two protein complexes in regulating the transforming activity of E1A are not well defined. Here, we report that the E1A N-terminal region also interacted with the cellular proto-oncoprotein c-MYC and the homolog of enhancer of yellow 2 (ENY2). Our results suggested that these proteins interacted with an essential E1A transforming domain spanning amino acid residues 26 to 35 which also interacted with TRRAP and p400. Small interfering RNA (siRNA)-mediated depletion of TRRAP reduced c-MYC interaction with E1A, while p400 depletion did not. In contrast, depletion of TRRAP enhanced ENY2 interaction with E1A, suggesting that ENY2 and TRRAP may interact with E1A in a competitive manner. The same E1A region additionally interacted with the constituents of a deubiquitinase complex consisting of USP22, ATXN7, and ATXN7L3 via TRRAP. Acute short hairpin RNA (shRNA)-mediated depletion of c-MYC reduced the E1A transforming activity, while depletion of ENY2 and MAX did not. These results suggested that the association of c-MYC with E1A may, at least partially, play a role in the E1A transformation activity, independently of MAX.The transforming region of adenovirus E1A consists of three short modules which complex with different cellular protein complexes. The mechanism by which one of the transforming modules, CR2, promotes cell proliferation, through inactivating the activities of the pRb family proteins, is better understood than the activities of the other domains. Our analysis of the E1A proteome revealed the presence of the proto-oncoprotein c-MYC and of ENY2. We mapped these interactions to a critical transforming module of E1A that was previously known to interact with the scaffolding molecule TRRAP and the E1A-binding protein p400. We showed that c-MYC interacted with E1A through TRRAP, while ENY2 interacted with it independently. The data reported here indicated that depletion of c-MYC in normal human cells reduced the transforming activity of E1A. Our result raises a novel paradigm in oncogenic transformation by a DNA viral oncogene, the E1A gene, that may exploit the activity of a cellular oncogene, the c-MYC gene, in addition to inactivation of the tumor suppressors, such as pRb. | Immunoblotting (Western), Immunoprecipitation | 26559831
 |
Chemical induction of unfolded protein response enhances cancer cell killing through lytic virus infection.
Prasad, V; Suomalainen, M; Pennauer, M; Yakimovich, A; Andriasyan, V; Hemmi, S; Greber, UF
Journal of virology
88
13086-98
2014
Mostra il sommario
Cancer cells are susceptible to oncolytic viruses, albeit variably. Human adenoviruses (HAdVs) are widely used oncolytic agents that have been engineered to produce progeny within the tumor and elicit bystander effects. We searched for host factors enhancing bystander effects and conducted a targeted RNA interference screen against guanine nucleotide exchange factors (GEFs) of small GTPases. We show that the unfolded protein response (UPR), which is readily inducible in aggressive tumor cells, enhances melanoma or epithelial cancer cell killing upon HAdV infection. UPR was triggered by knockdown of Golgi-specific brefeldin A-resistant guanine nucleotide exchange factor 1 (GBF-1) or the GBF-1 inhibitor golgicide A (GCA) and stimulated HAdV infection. GBF-1 is a GEF for ADP ribosylation factors (Arfs) regulating endoplasmic reticulum (ER)-to-Golgi apparatus and intra-Golgi apparatus membrane transport. Cells treated with GCA enhanced HAdV-induced cytopathic effects in epithelial and melanoma cancer cells but not normal cells, if the drug was applied several hours prior to HAdV inoculation. This was shown by real-time label-free impedance measurements using the xCELLigence system. GCA-treated cells contained fewer incoming HAdVs than control cells, but GCA treatment boosted HAdV titers and spreading in cancer cells. GCA enhanced viral gene expression or transgene expression from the cytomegalovirus promoter of B- or C-species HAdVs but did not enhance viral early region 1A (E1A) expression in uninfected cell lines or cells transfected with plasmid reporter DNA. The UPR-enhanced cell killing required the nuclease activity of the UPR sensor inositol-requiring enzyme 1 (IRE-1) and X box binding protein 1 (XBP-1), which alleviate ER stress. The collective results show that chemical UPR induction and viruses boost tumor cell killing by enhancing oncolytic viral efficacy.Cancer is difficult to combat. A wide range of oncolytic viruses show promise for killing cancer cells, yet the efficacy of oncolytic killing is low. We searched for host factors enhancing adenovirus cancer cell killing and found that the knockdown of Golgi-specific brefeldin A-resistant guanine nucleotide exchange factor 1 (GBF-1) or chemical inhibition of GBF-1 enhanced adenovirus infection by triggering the IRE-1/XBP-1 branch of the unfolded protein response (UPR). IRE-1/XBP-1 promote cell survival and enhanced the levels of the adenoviral immediate early gene product E1A, virus spreading, and killing of cancer cells. Aggressive tumor cells depend on a readily inducible UPR and, hence, present prime targets for a combined strategy involving adenoviruses and small chemicals inducing UPR. | | 25187554
 |
Adenovirus type 5 E1A and E6 proteins of low-risk cutaneous beta-human papillomaviruses suppress cell transformation through interaction with FOXK1/K2 transcription factors.
Jessica Komorek,Mohan Kuppuswamy,T Subramanian,S Vijayalingam,Elena Lomonosova,Ling-Jun Zhao,Joe S Mymryk,Kimberly Schmitt,G Chinnadurai
Journal of virology
84
2009
Mostra il sommario
The adenovirus (Adv) oncoprotein E1A stimulates cell proliferation and inhibits differentiation. These activities are primarily linked to the N-terminal region (exon 1) of E1A, which interacts with multiple cellular protein complexes. The C terminus (exon 2) of E1A antagonizes these processes, mediated in part through interaction with C-terminal binding proteins 1 and 2 (CtBP1/2). To identify additional cellular E1A targets that are involved in the modulation of E1A C-terminus-mediated activities, we undertook tandem affinity purification of E1A-associated proteins. Through mass spectrometric analysis, we identified several known E1A-interacting proteins as well as novel E1A targets, such as the forkhead transcription factors, FOXK1/K2. We identified a Ser/Thr-containing sequence motif in E1A that mediated interaction with FOXK1/K2. We demonstrated that the E6 proteins of two beta-human papillomaviruses (HPV14 and HPV21) associated with epidermodysplasia verruciformis also interacted with FOXK1/K2 through a motif similar to that of E1A. The E1A mutants deficient in interaction with FOXK1/K2 induced enhanced cell proliferation and oncogenic transformation. The hypertransforming activity of the mutant E1A was suppressed by HPV21 E6. An E1A-E6 chimeric protein containing the Ser/Thr domain of the E6 protein in E1A interacted efficiently with FOXK1/K2 and inhibited cell transformation. Our results suggest that targeting FOXK1/K2 may be a common mechanism for certain beta-HPVs and Adv5. E1A exon 2 mutants deficient in interaction with the dual-specificity kinases DYRK1A/1B and their cofactor HAN11 also induced increased cell proliferation and transformation. Our results suggest that the E1A C-terminal region may suppress cell proliferation and oncogenic transformation through interaction with three different cellular protein complexes: FOXK1/K2, DYRK(1A/1B)/HAN11, and CtBP1/2. Testo completo dell'articolo | | 20053746
 |
Midkine promoter-based conditionally replicative adenovirus therapy for midkine-expressing human pancreatic cancer.
Toyoda, E; Doi, R; Kami, K; Mori, T; Ito, D; Koizumi, M; Kida, A; Nagai, K; Ito, T; Masui, T; Wada, M; Tagawa, M; Uemoto, S
Journal of experimental & clinical cancer research : CR
27
30
2008
Mostra il sommario
To develop a novel therapeutic strategy for human pancreatic cancer using a midkine promoter-based conditionally replicating adenovirus.We examined midkine mRNA expression and midkine protein expression by seven human pancreatic cancer cell lines (AsPC-1, BxPC-3, CFPAC-1, HPAC, MIAPaCa-2, PANC-1, and Suit-2), as well as by non-cancerous pancreatic tissue and pancreatic cancers. Midkine promoter activity was measured in cancer cell lines by the dual luciferase reporter assay. Adenoviral transduction efficiency was assessed by fluorescent staining of cancer cell lines using adenovirus type 5 containing the green fluorescent protein gene (Ad5GFP). Replication of adenovirus type 5 containing the 0.6 kb midkne promoter (Ad5MK) was assessed by the detection of E1 protein in cancer cell lines. The cytotoxicity of Ad5MK for cancer cells was evaluated from the extent of growth inhibition after viral infection. Infection and replication were also assessed in nude mice with subcutaneous Suit-2 tumors by intratumoral injection of Ad5MK, Ad5GFP, or vehicle. E1a mRNA expression in the treated tumors and expression of the replication-specific adenoviral hexon protein were evaluated. Finally, the anti-tumor activity of Ad5MK against intraperitoneal xenografts of Suit-2 pancreatic cancer cells was examined after intraperitoneal injection of the virus.Both midkine mRNA expression and midkine protein expression were strong in AsPC-1 and CFPAC-1 cell liens, moderate in BxPC-3, HPAC, and Suit-2 cell lines, and weak in PANC-1 and MIAPaCa-2 cell lines. Expression of midkine mRNA was significantly stronger in pancreatic cancers than in non-cancerous pancreatic tissues. The relative luciferase activity mediated by the 0.6 kb midkne fragment in AsPC-1, PANC-1, and Suit-2 cell lines was approximately 6 to 20 times greater than that in midkne-negative MIAPaCa-2 cell lines. Pancreatic cancer cell lines exhibited a heterogeneous adenoviral transduction profile. E1A expression was higher in cell lines with strong midkine expression than in cell lines with weak midkine expression. Ad5MK showed much greater cytotoxicity for midkine-expressing Suit-2 and PANC-1 cell lines than for midkine-negative MIAPaCa-2 cell lines. In the Suit-2 subcutaneous xenograft model, expression of E1A was detected in Ad5MK-treated tumors, but not in untreated and Ad5GFP-treated tumors. In the Suit-2 intraperitoneal xenograft model, the Ad5MK group survived for significantly longer than the Ad5GFP, PBS, and untreated groups.Ad5MK has an anti-tumor effect against human pancreatic cancer cell lines that express midkine mRNA. Midkine promoter-based conditionally replicative adenovirus might be a promising new gene therapy for pancreatic cancer. | | 18717994
 |
Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production.
Nayak, R; Pintel, DJ
Journal of virology
81
2205-12
2007
Mostra il sommario
Full replication of adeno-associated virus type 5 (AAV5) is sustained by adenovirus type 5 (Ad5) helper functions E1a, E1b, E2a, E4Orf6, and virus-associated (VA) RNA; however, their combined net enhancement of AAV5 replication was comprised of both positive and negative individual effects. Although Ad5 E4Orf6 was required for AAV5 genomic DNA replication, it also functioned together with E1b to degrade de novo-expressed, preassembled AAV5 capsid proteins and Rep52 in a proteosome-dependent manner. VA RNA enhanced accumulation of AAV5 protein, overcoming the degradative effects of E4Orf6, and was thus required to restore adequate amounts of AAV5 proteins necessary to achieve efficient virus production. Testo completo dell'articolo | | 17166904
 |
The co-activator p300 associates physically with and can mediate the action of the distal enhancer of the FGF-4 gene♪Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor
Nowling, T., et al
J Biol Chem, 278:13696-705 (2003)
2003
| Immunoprecipitation | 12488456
 |
Inhibition of tumorigenicity of cervical cancer cells in nude mice by HPV E6-E7 anti-sense RNA.
M von Knebel Doeberitz,C Rittmüller,H zur Hausen,M Dürst
International journal of cancer. Journal international du cancer
51
1992
| | 1319412
 |
Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products
Harlow, E, et al
J Virol, 55:533-46 (1985)
1985
| | 3894685
 |