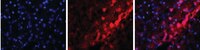
Aldosterone inhibits antifibrotic factors in mouse hypertensive heart.
Azibani, Feriel, et al.
Hypertension, 59: 1179-87 (2012)
2011
Mostra il sommario
The renin-angiotensin-aldosterone system is involved in the arterial hypertension-associated cardiovascular remodeling. In this context, the development of cardiac fibrosis results from an imbalance between profibrotic and antifibrotic pathways, in which the role of aldosterone is yet not established. To determine the role of intracardiac aldosterone in the development of myocardial fibrosis during hypertension, we used a double transgenic model (AS-Ren) of cardiac hyperaldosteronism (AS) and systemic hypertension (Ren). The 9-month-old hypertensive mice had cardiac fibrosis, and hyperaldosteronism enhanced the fibrotic level. The mRNA levels of connective tissue growth factor and transforming growth factor-β1 were similarly increased in Ren and AS-Ren mice compared with wild-type and AS mice, respectively. Hyperaldosteronism combined with hypertension favored the macrophage infiltration (CD68(+) cells) in heart, and enhanced the mRNA level of monocyte chemoattractant protein 1, osteopontin, and galectin 3. Interestingly, in AS-Ren mice the hypertension-induced increase in bone morphogenetic protein 4 mRNA and protein levels was significantly inhibited, and B-type natriuretic peptide expression was blunted. The mineralocorticoid receptor antagonist eplerenone restored B-type natriuretic peptide and bone morphogenetic protein 4 levels and decreased CD68 and galectin 3 levels in AS-Ren mice. Finally, when hypertension was induced by angiotensin II infusion in wild-type and AS mice, the mRNA profiles did not differ from those observed in Ren and AS-Ren mice, respectively. The aldosterone-induced inhibition of B-type natriuretic peptide and bone morphogenetic protein 4 expression was confirmed in vitro in neonatal mouse cardiomyocytes. Altogether, we demonstrate that, at the cardiac level, hyperaldosteronism worsens hypertension-induced fibrosis through 2 mineralocorticoid receptor-dependent mechanisms, activation of inflammation/galectin 3-induced fibrosis and inhibition of antifibrotic factors (B-type natriuretic peptide and bone morphogenetic protein 4). | 22547442
 |
[Expression of BMP4 mature peptide in eukaryotic cells and its differentiation-inhibiting effect in culturing induced pluripotent stem cells].
Dao-Fang Ding,Zhen Wang,Hao Xu,Le-Qin Xu,Qian-Qian Liang,Yong-Jian Zhao,Qi Shi,Yong-Jun Wang
Nan fang yi ke da xue xue bao = Journal of Southern Medical University
32
2011
Mostra il sommario
To investigate the role of bone morphogenetic protein 4 (BMP4) in culturing induced pluripotent stem cells (iPSCs) and the related signal pathways. | 23076169
 |
The bone morphogenetic protein antagonist noggin protects white matter after perinatal hypoxia-ischemia.
Dizon ML, Maa T, Kessler JA
Neurobiol Dis
2010
Mostra il sommario
Hypoxia-ischemia (HI) in the neonate leads to white matter injury and subsequently cerebral palsy. We find that expression of bone morphogenetic protein 4 (BMP4) increases in the neonatal mouse brain after unilateral common carotid artery ligation followed by hypoxia. Since signaling by the BMP family of factors is a potent inhibitor of oligodendroglial differentiation, we tested the hypothesis that antagonism of BMP signaling would prevent loss of oligodendroglia (OL) and white matter in a mouse model of perinatal HI. Perinatal HI was induced in transgenic mice in which the BMP antagonist noggin is overexpressed during oligodendrogenesis (pNSE-Noggin). Following perinatal HI, pNSE-Noggin mice had more oligodendroglial progenitor cells (OPCs) and more mature OL compared to wild type (WT) animals. The increase in OPC numbers did not result from proliferation but rather from increased differentiation from precursor cells. Immunofluorescence studies showed preservation of white matter in lesioned pNSE-Noggin mice compared to lesioned WT animals. Further, following perinatal HI, the pNSE-Noggin mice were protected from gait deficits. Together these findings indicate that the BMP-inhibitor noggin protects from HI-induced loss of oligodendroglial lineage cells and white matter as well as loss of motor function.Copyright © 2011 Elsevier Inc. All rights reserved. | 21310236
 |
Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis.
Crews L, Adame A, Patrick C, Delaney A, Pham E, Rockenstein E, Hansen L, Masliah E.
The Journal of Neuroscience, 2010 Sep 15;30(37)
2009
Mostra il sommario
During aging and in the progression of Alzheimer's disease (AD), synaptic plasticity and neuronal integrity are disturbed. In addition to the alterations in plasticity in mature neurons, the neurodegenerative process in AD has been shown to be accompanied by alterations in neurogenesis. Members of the bone morphogenetic protein (BMP) family of growth factors have been implicated as important regulators of neurogenesis and neuronal cell fate determination during development; however, their role in adult neurogenesis and in AD is less clear. We show here by qRT-PCR analysis that BMP6 mRNA levels were significantly increased in the hippocampus of human patients with AD and in APP transgenic mice compared to controls. Immunoblot and immunohistochemical analyses confirmed that BMP6 protein levels were increased in human AD brains and APP transgenic mouse brains compared to controls and accumulated around hippocampal plaques. The increased levels of BMP6 were accompanied by defects in hippocampal neurogenesis in AD patients and APP transgenic mice. In support of a role for BMP6 in defective neurogenesis in AD, we show in an in vitro model of adult neurogenesis that treatment with amyloid-β(1-42) protein (Aβ) resulted in increased expression of BMP6, and that exposure to recombinant BMP6 resulted in reduced proliferation with no toxic effects. Together, these results suggest that Aβ-associated increases in BMP6 expression in AD may have deleterious effects on neurogenesis in the hippocampus, and therapeutic approaches could focus on normalization of BMP6 levels to protect against AD-related neurogenic deficits. | 20844121
 |
GATA2 and Sp1 Positively Regulate the c-kit Promoter in Mast Cells.
Maeda K, Nishiyama C, Ogawa H, Okumura K
J Immunol
185
4252-60. Epub 2010 Sep 10.
2009
Mostra il sommario
The c-kit gene is expressed in hematopoietic stem cells and lineage progenitor cells but is downregulated during cell development in most lineages, except for mast cells. In mast cells, high expression of c-kit is maintained during development, and c-Kit signaling is essential for mast cell development. To analyze the mechanisms by which c-kit gene expression are regulated in mast cells, we examined mast cell type-specific regulation of the c-kit promoter region. We observed that a GC-box in the c-kit promoter was critical for transcriptional activity and was bound to the transcription factor Sp1 as assessed using reporter assay and electrophoretic mobility assay. Chromatin immunoprecipitation assay and coexpression analyses showed that the transcription factor GATA2, which was recruited to the c-kit promoter in a mast cell-specific manner, in addition to Sp1, transactivated the c-kit promoter via the GC-box. Electrophoretic mobility assay and rechromatin immunoprecipitation assay indicated that GATA2 binds to the GC-box by forming a complex with Sp1. Introduction of Sp1 small interfering RNA significantly reduced the amount not only of Sp1 but also of GATA2 binding to the c-kit promoter in mast cells, resulting in suppression of c-kit transcription. Knockdown of GATA2 suppressed the recruitment of GATA2 toward the c-kit promoter, subsequently suppressing cell surface expression of c-Kit. These findings indicate that GATA2 and Sp1 play crucial roles in expression of the c-kit gene in mast cells. | 20833840
 |
mDia1 targets v-Src to the cell periphery and facilitates cell transformation, tumorigenesis, and invasion.
Tanji M, Ishizaki T, Ebrahimi S, Tsuboguchi Y, Sukezane T, Akagi T, Frame MC, Hashimoto N, Miyamoto S, Narumiya S
Mol Cell Biol
30
4604-15. Epub 2010 Aug 2.
2009
Mostra il sommario
The small GTPase Rho regulates cell morphogenesis through remodeling of the actin cytoskeleton. While Rho is overexpressed in many clinical cancers, the role of Rho signaling in oncogenesis remains unknown. mDia1 is a Rho effector producing straight actin filaments. Here we transduced mouse embryonic fibroblasts from mDia1-deficient mice with temperature-sensitive v-Src and examined the involvement and mechanism of the Rho-mDia1 pathway in Src-induced oncogenesis. We showed that in v-Src-transduced mDia1-deficient cells, formation of actin filaments is suppressed, and v-Src in the perinuclear region does not move to focal adhesions upon a temperature shift. Consequently, membrane translocation of v-Src, v-Src-induced morphological transformation, and podosome formation are all suppressed in mDia1-deficient cells with impaired tyrosine phosphorylation. mDia1-deficient cells show reduced transformation in vitro as examined by focus formation and colony formation in soft agar and exhibit suppressed tumorigenesis and invasion when implanted in nude mice in vivo. Given overexpression of c-Src in various cancers, these findings suggest that Rho-mDia1 signaling facilitates malignant transformation and invasion by manipulating the actin cytoskeleton and targeting Src to the cell periphery. | 20679479
 |
Transient Overexpression of Gremlin Results in Epithelial Activation and Reversible Fibrosis in Rat Lungs.
Farkas L, Farkas D, Gauldie J, Warburton D, Shi W, Kolb M
Am J Respir Cell Mol Biol
2009
Mostra il sommario
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic disease of the lung parenchyma, without curative treatment. Gremlin is a bone morphogenic protein (BMP) antagonist, its expression being increased in IPF lungs. It has been implicated in promoting myofibroblast accumulation, likely through inhibited fibroblast apoptosis and epithelial-to-mesenchymal transition (EMT). In the current study we examined the effects of selective adenovirus-mediated overexpression of Gremlin in rat lungs. We show that transient Gremlin overexpression results in activation of alveolar epithelial cells with proliferation and apoptosis, as well as partly reversible lung fibrosis. We found myofibroblasts arranged in fibroblastic foci, together with evidence for EMT. Fibroblast proliferation occurred delayed as compared to epithelial changes. Fibrotic pathology significantly declined after day 14, the reversal being associated with an increase of the epithelial protective element fibroblast growth factor 10 (FGF10). Our data indicate that Gremlin-mediated BMP inhibition results in activation of epithelial cells and transient fibrosis, but also induction of epithelium protective FGF10. A Gremlin-BMP-FGF10 loop may explain these results, and demonstrate that the interaction between different factors are quite complex in fibrotic lung disease. Increased Gremlin expression in human IPF tissue may be an expression of continuing epithelial injury, and Gremlin may be part of activated repair mechanisms. | 20705941
 |
Gremlin localization and expression levels partially differentiate idiopathic interstitial pneumonia severity and subtype.
M Myllärniemi, K Vuorinen, V Pulkkinen, H Kankaanranta, T Aine, K Salmenkivi, J Keski-Oja, K Koli, Vl Kinnula
The Journal of pathology
214
456-63
2008
Mostra il sommario
Idiopathic pulmonary fibrosis (IPF) (histopathology of usual interstitial pneumonia, UIP) and non-specific interstitial pneumonia (NSIP) are diseases characterized by loss of normal lung architecture and function. The differential diagnosis between IPF/UIP and NSIP may be difficult. The levels of bone morphogenetic protein (BMP)-4 antagonist gremlin are up-regulated in IPF/UIP. The present study was performed to clarify whether the localization or the mRNA expression of gremlin or BMP-4 could be used in the differential diagnosis or assessment of severity of IPF/UIP and NSIP. Gremlin and BMP-4 immunoreactivities were quantitated from 24 UIP and 12 NSIP lung specimens. Quantitative real-time polymerase chain reaction analyses were performed to compare gremlin and BMP-4 expression between UIP (n = 8) and NSIP (n = 5) biopsies. Immunohistochemical positivity and mRNA levels were correlated to lung function parameters. In IPF/UIP biopsies, gremlin was detected mainly in the thickened lung parenchyma, whereas in NSIP it was observed in the alveolar epithelium. BMP-4-positive (BMP-4+) cells were detected solely in the alveolar wall. The percentage of gremlin-positive area was higher in IPF/UIP (5.1 +/- 0.6) than in NSIP (1.8 +/- 0.7) (n = 36, p 0.0001). Gremlin mRNA levels were higher in advanced UIP (p = 0.008) and NSIP (p = 0.007) biopsies than in the normal control lung. A negative correlation was found between the specific diffusion capacity corrected for alveolar volume (DLCO/VA) and gremlin mRNA levels (r = - 0.69, p = 0.007). The highest numbers of BMP-4+ cells were found in NSIP biopsies. BMP-4 mRNA levels correlated positively with forced vital capacity (r = 0.801, p 0.0001) and diffusion capacity. Parenchymal gremlin immunoreactivity is thus suggestive of a UIP-type interstitial pneumonia. Gremlin expression levels correlating negatively and BMP-4 levels positively with disease severity support recent observations of a fibroprotective role for the BMPs. | 18072275
 |
Activation of the BMP canonical signaling pathway in human optic nerve head tissue and isolated optic nerve head astrocytes and lamina cribrosa cells.
Zode, GS; Clark, AF; Wordinger, RJ
Investigative ophthalmology & visual science
48
5058-67
2007
Mostra il sommario
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor (TGF)-beta superfamily that controls multiple functions in a variety of cells. We have previously shown that human optic nerve head (ONH) astrocytes and lamina cribrosa (LC) cells express BMP and BMP receptor mRNA and proteins. The purpose of the present study was to determine whether human ONH tissues express the canonical BMP signaling pathway and whether ONH cells secrete BMP-4 and respond to exogenous BMP-4 through this pathway.Well-characterized human ONH astrocytes (N = 2) and LC cells (N = 3) were treated with exogenous BMP-4 (20 ng/mL) for various times. Western immunoblot analysis was used to detect secreted BMP-4 in serum-free conditioned media of ONH cells and in human ONH tissues (N = 4) and Smad proteins in total cell lysate of ONH cells and tissues. Intracellular colocalization of p-R-Smad1 with Co-Smad4 and localization of inhibitory Smads (e.g., I-Smad6 and I-Smad7) were studied through immunocytochemistry. In addition, coimmunoprecipitation was used to verify the interaction of p-R-Smad1 with Co-Smad4.ONH astrocytes and LC cells secrete BMP-4 and synthesize R-Smad1, R-Smad5, I-Smad6, I-Smad7, and Co-Smad4 proteins. Exposure to BMP-4 for either 10 or 60 minutes resulted in increased p-R-Smad1 and p-R-Smad1/5/8 protein levels that declined after 12 hours of treatment. Immunocytochemistry and coimmunoprecipitation studies revealed that p-R-Smad1/5/8 and Co-Smad4 interact and colocalize in the nucleus. BMP-4 treatment resulted in increased coprecipitation of p-R-Smad1/5/ 8 and Co-Smad4. I-Smad6 and I-Smad7 are localized in the nucleus and cytoplasm of ONH astrocytes and LC cells. Proteins for BMP-4, p-R-Smad1/5/8, R-Smad1, R-Smad5, R-Smad8, and Co-Smad4 are present in human ONH tissues. In addition, phosphorylated Smad1 and Smad5 colocalize with Smad4 in the nuclei of ONH tissues.These results indicate that BMP-4 and Smad signaling proteins are present in human ONH tissues, isolated ONH astrocytes, and LC cells. In addition, exogenous BMP-4 treatment of ONH astrocytes and LC cells results in downstream signaling through the canonical Smad pathway. Thus, cells within the human ONH may respond to locally released BMP through paracrine or autocrine mechanisms. | 17962458
 |
Use of monoclonal antibody to detect bone morphogenetic protein-4 (BMP-4).
Masuhara, K, et al.
Bone, 16: 91-6 (1995)
1994
Mostra il sommario
A monoclonal antibody that reacts with murine and human bone morphogenetic protein-4 (BMP-4) has been developed using recombinant BMP-4 as an immunogen. The antibody that bound most tightly to recombinant murine (rm)BMP-4 was selected, subcloned, and characterized. The specificity of the antibody was confirmed using Western blot analysis and enzyme-linked immunosorbent assay (ELISA). The antibody reacts with murine and human BMP-4 in both the reduced and nonreduced condition; however, this antibody shows cross-reactivity with neither human BMP-2 nor TGF-beta 1. Thus, the produced antibody could recognize the disulfide-linked dimeric structure of bioactive BMP-4, regardless of the species. Immunocytochemical study using this antibody successfully shows the cytosolic localization of BMP-4 in osteoinductive cells; i.e., BFO and Saos-2 in which the level of mRNA for BMP-4 was proved to be constitutively high by Northern blot analysis. In addition, the antibody could demonstrate the presence of BMP-4 in developmental bone formation in the alveolar bone of rat embryo by immunohistochemistry. The antibody could be used for a more sensitive approach for quantitative analysis of BMP-4. | 7742091
 |