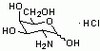
| Corinne D. Hausmann, Mette Prætorius-Ibba and Michael Ibba. (2007) An aminoacyl-tRNA synthetase:elongation factor complex for substrate channeling in archaeal translation. Nucleic Acids Research 35, 6094-6102.
James B. Ames, et al. (2006) Structural basis for calcium-induced inhibition of rhodopsin kinase by recoverin. Journal of Biological Chemistry 281, 37237-37245.
Alex Kudrin, et al. (2006) Human macrophage migration inhibitory factor: a proven immumodulatory cytokine? Journal of Biological Chemistry 281, 29641-29651.
Anastasia Metlitskaya, et al. (2006) Aspartyl-tRNA synthetase is the target of peptide nucleotide antibiotic microcin C. Journal of Biological Chemistry 281, 18033-18042.
Hiroshi M. Sasaki, et al. (2006) Structural and mutational studies of the amino acid-editing domain from archaeal/eukaryal phenylalanyl-tRNA synthetase. Procedings of the National Academy of Science 103, 14744-14749.
Hong Ye, et al. (2006) CpSufE activates the cysteine desulfurase CpNifS for chloroplastic Fe-S cluster formation. Journal of Biological Chemistry 281, 8958-8969.
Sarae L. Bausch, Ekaterina Poliakova and David E. Draper. (2005) Interactions of the N-terminal domain of ribosomal protein L11 with thiostrepton and rRNA. Journal of Biological Chemistry 280, 29956-29963.
Michaël Boyer-Guittaut, et al. (2005) Sumo-1 modification of human TFIID complex subunits: Inhibition of TFIID promoter binding activity through sumo-1 modification of hsTAF5. Journal of Biological Chemistry 280, 9937-9945.
Giulia Calloni, et al. (2005) Investigating the effects of mutations on protein aggregation in the cell. Journal of Biological Chemistry 280, 10607-10613.
Jessica C. Greene, et al. (2005) Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Human Molecular Genetics 14, 799-811.
Noriko Handa, et al. (2005) Crystal structure of a novel polyisoprenoid-binding protein from Thermus thermophilus HB8. Protein Science 14, 1004-1010.
Valerie Heurgue-Hamard, et al. (2005) The glutamine residue of the conserved GGQ motif in Saccharomyces cerevisiae release factor eRF1 Is methylated by the product of the YDR140w gene. Journal of Biological Chemistry 280, 2439-2445.
Frank Hillger, et al. (2005) Biophysical comparison of BMP-2, ProBMP-2, and the free pro-peptide reveals stabilization of the pro-peptide by the mature growth factor. Journal of Biological Chemistry 280, 14974-14980.
Mette Praetorius Ibba, et al. (2005) Association between archaeal prolyl-and leucyl-tRNA synthetases enhances tRNAPro aminoacylation. Journal of Biological Chemistry 280, 26099-26104.
Eva Johansson, et al. (2005) Structures of dCTP deaminase from Escherichia coli with bound substrate and product: reaction mechanism and determinants of mono- and bifunctionality for a family of enzymes. Journal of Biological Chemistry 280, 3051-3059.
Peter A. Lemaire, Jeffrey Lary and James L. Cole. (2005) Mechanism of PKR activation: dimerization and kinase activation in the absence of double-stranded RNA. Journal of Molecular Biology 345, 81-90.
Mette Praetorius-Ibba, et al. (2005) Association between archaeal prolyl- and leucyl-tRNA synthetases enhances tRNApro aminoacylation. Journal of Biological Chemistry 280, 26099-26104.
Rongde Qiu, et al. (2005) Identification of the putative staphylococcal AgrB catalytic residues involving in the proteolytic cleavage of AgrD to generate autoinducing peptide. Journal of Biological Chemistry 280, 16695-16704.
Ok Hee Ryu, et al. (2005) Proteolysis of MIP-1α isoforms LD78β and LD78α by neutrophil-derived serine proteases. Journal of Biological Chemistry 280, 17415-17421.
D. Andrew Skaff, et al. (2005) Glucose 6-phosphate release of wild-type and mutant human brain hexokinases from mitochondria. Journal of Biological Chemistry 280, 38403-38409.
Wayne W. Chan, Steven L. Roderick and David E. Cohen. (2002) Human phosphatidylcholine transfer protein: purification, crystallization and preliminary X-ray diffraction data. Biochimica et Biophysica Acta 1596, 1-5.
Angelina J. Lay, et al. (2000) Phosphoglycerate kinase acts in tumor angiogenesis as a disulphide reductase. 408, 869-873.
Jianyuan Luo, et al. (2000) Deactylation of p53 modul;ates its effect on cell growth and apoptosis. 408, 377-381.
G.L. Conn, et al. (1999) Crystal structure of a conserved ribosomal protein-RNA complex. Science 284, 1171-1174.
Trudy H. Grossman, et al. (1998) Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 209, 95-103.
C. C. Hu, et al. (1997) Cloning, characterization, and heterologous expression of exon-4-containing amelogenin mRNAs. Journal of Dental Research 76, 641-647.
Ren Zhang, JiaJu Zhao and James D. Potter. (1995) Phosphorylation of both serine residues in cardiac troponin I is required to decrease the Ca2+ affinity of cardiac troponin C. Journal of Biological Chemistry 270, 30773-30780.
I. Aukhil, et al. (1993) Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. Journal of Biological Chemistry 268, 2542-2553.
K.M. Bohren, et al. (1991) Expression of human aldose and aldehyde erductases. Journal of Biological Chemistry 266, 24031-24037.
|
|