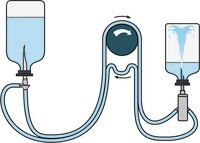
Steritest™ NEO Device for products requiring increased Chemical Compatibility
Green canister base with specific drain design. Low binding PVDF Durapore® membrane optimize the rinsing of products that inhibit microbial growth. Canisters material: polyamide polymer.
The Steritest™ NEO Device for sterility testing of solvents, creams, ointments and veterinary injectables includes:
- Canister designed for testing products dissolved in solvents such as Isopropyl Myristate
- Canister connections and reinforced base structure provide better resistance to pressure
- Single needle adapter for products in vials or ampoules
- Separate vent needle
The Steritest™ NEO Device for sterility testing of solvents, creams, ointments and veterinary injectables includes:
- Canister designed for testing products dissolved in solvents such as Isopropyl Myristate
- Canister connections and reinforced base structure provide better resistance to pressure
- Single needle adapter for products in vials or ampoules
- Separate vent needle
Less<<
Documents associés
Produits recommandés
Aperçu
Caractéristiques
Guide d'achat
Documentation
Références bibliographiques
| Aperçu de la référence bibliographique | Nº PubMed |
|---|---|
| The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices Roche Lentine, Kerry and Robert J. Keller; Pharmaceutical Technology Europe, February 2003: 31- 42. Pharmaceutical Technology Europe, February 2003: 31- 42. 2003 | The Effect of Vaporous Phase Hydrogen Peroxide on Sterility Test Devices
 |