220285 Sigma-AldrichChelerythrine Chloride - CAS 3895-92-9 - Calbiochem
Produits recommandés
Aperçu
| Replacement Information |
|---|
Tableau de caractéristiques principal
| CAS # | Empirical Formula |
|---|---|
| 3895-92-9 | C₂₁H₁₈NO₄Cl |
Prix & Disponibilité
| Référence | Disponibilité | Conditionnement | Qté | Prix | Quantité | |
|---|---|---|---|---|---|---|
| 220285-5MG |
|
Ampoule plast. | 5 mg |
|
— |
| Description | |
|---|---|
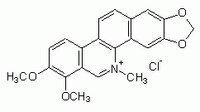
| Overview | Naturally-occurring alkaloid. Potent, selective, and cell-permeable inhibitor of protein kinase C (IC50 = 660 nM). Acts on the catalytic domain of PKC. A competitive inhibitor with respect to the phosphate acceptor and a non-competitive inhibitor with respect to ATP. Over ten-fold more potent than H-7, HCl (Cat. No. 371955). Inhibits thromboxane formation and phosphoinositide metabolism in platelets. Also induces apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells. |
| Catalogue Number | 220285 |
| Brand Family | Calbiochem® |
| Product Information | |
|---|---|
| CAS number | 3895-92-9 |
| ATP Competitive | N |
| Form | Light yellow to yellow solid |
| Hill Formula | C₂₁H₁₈NO₄Cl |
| Chemical formula | C₂₁H₁₈NO₄Cl |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PKC |
| Primary Target IC<sub>50</sub> | 660 nM |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | FL9200000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Référence | GTIN |
| 220285-5MG | 07790788048761 |
Documentation
Required Licenses
| Title |
|---|
| PRODUCTO REGULADO POR LA SECRETARÍA DE SALUD |
Chelerythrine Chloride - CAS 3895-92-9 - Calbiochem FDS
| Titre |
|---|
Chelerythrine Chloride - CAS 3895-92-9 - Calbiochem Certificats d'analyse
| Titre | Numéro de lot |
|---|---|
| 220285 |
Références bibliographiques
| Aperçu de la référence bibliographique |
|---|
| Kandasamy, R.A., et al. 1995. J. Biol. Chem. 270 29209. Jarvis, W.D., et al. 1994. Cancer Res. 54, 1707. Barg, J., et al. 1992. J. Neurochem. 59, 1145. Herbert, J.M., et al. 1990. Biochem. Biophys. Res. Commun. 172, 993. Ko, F., et al. 1990. Biochim. Biophys. Acta 1052, 360. Walterova, D., et al. J. Med. Chem. 24, 1100. |