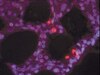
Identification of novel and distinct binding sites within tenascin-C for soluble and fibrillar fibronectin.
To, WS; Midwood, KS
The Journal of biological chemistry
286
14881-91
2010
Afficher le résumé
Interactions between fibronectin and tenascin-C within the extracellular matrix provide specific environmental cues that dictate tissue structure and cell function. The major binding site for fibronectin lies within the fibronectin type III-like repeats (TNfn) of tenascin-C. Here, we systematically screened TNfn domains for their ability to bind to both soluble and fibrillar fibronectin. All TNfn domains containing the TNfn3 module interact with soluble fibronectin. However, TNfn domains bind differentially to fibrillar fibronectin. This distinct binding pattern is dictated by the fibrillar conformation of FN. TNfn1-3, but not TNfn3-5, binds to immature fibronectin fibrils, and additional TNfn domains are required for binding to mature fibrils. Multiple binding sites for distinct regions of fibronectin exist within tenascin-C. TNfn domains comprise a binding site for the N-terminal 70-kDa domain of fibronectin that is freely available and a binding site for the central binding domain of fibronectin that is cryptic in full-length tenascin-C. The 70-kDa and central binding domain regions are key for fibronectin matrix assembly; accordingly, binding of several TNfn domains to these regions inhibits fibronectin fibrillogenesis. These data highlight the complexity of protein-protein binding, the importance of protein conformation on these interactions, and the implications for the physiological assembly of complex three-dimensional matrices. | 21324901
 |