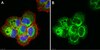
Synaptic dysregulation in a human iPS cell model of mental disorders.
Wen, Z; Nguyen, HN; Guo, Z; Lalli, MA; Wang, X; Su, Y; Kim, NS; Yoon, KJ; Shin, J; Zhang, C; Makri, G; Nauen, D; Yu, H; Guzman, E; Chiang, CH; Yoritomo, N; Kaibuchi, K; Zou, J; Christian, KM; Cheng, L; Ross, CA; Margolis, RL; Chen, G; Kosik, KS; Song, H; Ming, GL
Nature
2014
Afficher le résumé
Dysregulated neurodevelopment with altered structural and functional connectivity is believed to underlie many neuropsychiatric disorders, and 'a disease of synapses' is the major hypothesis for the biological basis of schizophrenia. Although this hypothesis has gained indirect support from human post-mortem brain analyses and genetic studies, little is known about the pathophysiology of synapses in patient neurons and how susceptibility genes for mental disorders could lead to synaptic deficits in humans. Genetics of most psychiatric disorders are extremely complex due to multiple susceptibility variants with low penetrance and variable phenotypes. Rare, multiply affected, large families in which a single genetic locus is probably responsible for conferring susceptibility have proven invaluable for the study of complex disorders. Here we generated induced pluripotent stem (iPS) cells from four members of a family in which a frameshift mutation of disrupted in schizophrenia 1 (DISC1) co-segregated with major psychiatric disorders and we further produced different isogenic iPS cell lines via gene editing. We showed that mutant DISC1 causes synaptic vesicle release deficits in iPS-cell-derived forebrain neurons. Mutant DISC1 depletes wild-type DISC1 protein and, furthermore, dysregulates expression of many genes related to synapses and psychiatric disorders in human forebrain neurons. Our study reveals that a psychiatric disorder relevant mutation causes synapse deficits and transcriptional dysregulation in human neurons and our findings provide new insight into the molecular and synaptic etiopathology of psychiatric disorders. | | 25132547
 |
PDE5 inhibition improves object memory in standard housed rats but not in rats housed in an enriched environment: implications for memory models?
Akkerman, S; Prickaerts, J; Bruder, AK; Wolfs, KH; De Vry, J; Vanmierlo, T; Blokland, A
PloS one
9
e111692
2014
Afficher le résumé
Drug effects are usually evaluated in animals housed under maximally standardized conditions. However, it is assumed that an enriched environment (EE) more closely resembles human conditions as compared to maximally standardized laboratory conditions. In the present study, we examined the acute cognition enhancing effects of vardenafil, a PDE5 inhibitor, which stimulates protein kinase G/CREB signaling in cells, in three different groups of male Wistar rats tested in an object recognition task (ORT). Rats were either housed solitarily (SOL) or socially (SOC) under standard conditions, or socially in an EE. Although EE animals remembered object information longer in the vehicle condition, vardenafil only improved object memory in SOL and SOC animals. While EE animals had a heavier dorsal hippocampus, we found no differences between experimental groups in total cell numbers in the dentate gyrus, CA2-3 or CA1. Neither were there any differences in markers for pre- and postsynaptic density. No changes in PDE5 mRNA- and protein expression levels were observed. Basal pCREB levels were increased in EE rats only, whereas β-catenin was not affected, suggesting specific activation of the MAP kinase signaling pathway and not the AKT pathway. A possible explanation for the inefficacy of vardenafil could be that CREB signaling is already optimally stimulated in the hippocampus of EE rats. Since previous data has shown that acute PDE5 inhibition does not improve memory performance in humans, the use of EE animals could be considered as a more valid model for testing cognition enhancing drugs. | | 25372140
 |
Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1.
Gokhale, A; Larimore, J; Werner, E; So, L; Moreno-De-Luca, A; Lese-Martin, C; Lupashin, VV; Smith, Y; Faundez, V
The Journal of neuroscience : the official journal of the Society for Neuroscience
32
3697-711
2011
Afficher le résumé
The Biogenesis of Lysosome-Related Organelles Complex 1 (BLOC-1) is a protein complex containing the schizophrenia susceptibility factor dysbindin, which is encoded by the gene DTNBP1. However, mechanisms engaged by dysbindin defining schizophrenia susceptibility pathways have not been quantitatively elucidated. Here, we discovered prevalent and novel cellular roles of the BLOC-1 complex in neuronal cells by performing large-scale Stable Isotopic Labeling of Cells in Culture (SILAC) quantitative proteomics combined with genetic analyses in dysbindin-null mice (Mus musculus) and the genome of schizophrenia patients. We identified 24 proteins that associate with the BLOC-1 complex, many of which were altered in content/distribution in cells or tissues deficient in BLOC-1. New findings include BLOC-1 interactions with the COG complex, a Golgi apparatus tether, and antioxidant enzymes peroxiredoxins 1-2. Importantly, loci encoding eight of the 24 proteins are affected by genomic copy number variation in schizophrenia patients. Thus, our quantitative proteomic studies expand the functional repertoire of the BLOC-1 complex and provide insight into putative molecular pathways of schizophrenia susceptibility. | Immunofluorescence | 22423091
 |
Mutant SOD1 impairs axonal transport of choline acetyltransferase and acetylcholine release by sequestering KAP3.
Tateno, M; Kato, S; Sakurai, T; Nukina, N; Takahashi, R; Araki, T
Human molecular genetics
18
942-55
2009
Afficher le résumé
Mutations in the superoxide dismutase 1 (sod1) gene cause familial amyotrophic lateral sclerosis (FALS), likely due to the toxic properties of misfolded mutant SOD1 protein. Here we demonstrated that, starting from the pre-onset stage of FALS, misfolded SOD1 species associates specifically with kinesin-associated protein 3 (KAP3) in the ventral white matter of SOD1(G93A)-transgenic mouse spinal cord. KAP3 is a kinesin-2 subunit responsible for binding to cargos including choline acetyltransferase (ChAT). Motor axons in SOD1(G93A)-Tg mice also showed a reduction in ChAT transport from the pre-onset stage. By employing a novel FALS modeling system using NG108-15 cells, we showed that microtubule-dependent release of acetylcholine was significantly impaired by misfolded SOD1 species. Furthermore, such impairment was able to be normalized by KAP3 overexpression. KAP3 was incorporated into SOD1 aggregates in human FALS cases as well. These results suggest that KAP3 sequestration by misfolded SOD1 species and the resultant inhibition of ChAT transport play a role in the dysfunction of ALS. | Western Blotting | 19088126
 |
ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2.
Tarr, Paul T and Edwards, Peter A
J. Lipid Res., 49: 169-82 (2008)
2008
Afficher le résumé
Here, we describe the initial characterization of Abcg4(-/-) mice and identify overlapping functions of ABCG4 and ABCG1 in the brain. Histological examination of tissues from Abcg4(+/-)/nlsLacZ and Abcg1(+/-)/nlsLacZ mice demonstrates that coexpression of Abcg4 and Abcg1 is restricted to neurons and astrocytes of the central nervous system (CNS). Interestingly, Abcg4 mRNA is undetectable outside the CNS, in contrast with the broad tissue and cellular expression of Abcg1. We also used primary astrocytes, microglia, neurons, and macrophages to demonstrate that the expression of Abcg1, but not Abcg4, is induced after the activation of liver X receptor. Cellular localization studies demonstrated that both proteins reside in RhoB-positive endocytic vesicle membranes. Furthermore, overexpression of either ABCG1 or ABCG4 increased the processing of sterol-regulatory element binding protein 2 (SREBP-2) to the transcriptionally active protein, thus accounting for the observed increase in the expression of SREBP-2 target genes and cholesterol synthesis. Consistent with these latter results, we show that the expression levels of the same SREBP-2 target genes are repressed in the brains of Abcg1(-/-) and, to a lesser extent, Abcg4(-/-) mice. Based on the results of the current study, we propose that ABCG1 and ABCG4 mediate the intracellular vesicular transport of cholesterol/sterols within both neurons and astrocytes to regulate cholesterol transport in the brain. | | 17916878
 |
Phosphatidylinositol-4-kinase type II alpha is a component of adaptor protein-3-derived vesicles
Salazar, Gloria, et al
Mol Biol Cell, 16:3692-704 (2005)
2004
| | 15944223
 |
Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture.
Thomas C Schulz, Scott A Noggle, Gail M Palmarini, Deb A Weiler, Ian G Lyons, Kate A Pensa, Adrian C B Meedeniya, Bruce P Davidson, Nevin A Lambert, Brian G Condie
Stem cells (Dayton, Ohio)
22
1218-38
2004
Afficher le résumé
The use of human embryonic stem cells (hESCs) as a source of dopaminergic neurons for Parkinson's disease cell therapy will require the development of simple and reliable cell differentiation protocols. The use of cell cocultures, added extracellular signaling factors, or transgenic approaches to drive hESC differentiation could lead to additional regulatory as well as cell production delays for these therapies. Because the neuronal cell lineage seems to require limited or no signaling for its formation, we tested the ability of hESCs to differentiate to form dopamine-producing neurons in a simple serum-free suspension culture system. BG01 and BG03 hESCs were differentiated as suspension aggregates, and neural progenitors and neurons were detectable after 2-4 weeks. Plated neurons responded appropriately to electrophysiological cues. This differentiation was inhibited by early exposure to bone morphogenic protein (BMP)-4, but a pulse of BMP-4 from days 5 to 9 caused induction of peripheral neuronal differentiation. Real-time polymerase chain reaction and whole-mount immunocytochemistry demonstrated the expression of multiple markers of the midbrain dopaminergic phenotype in serum-free differentiations. Neurons expressing tyrosine hydroxylase (TH) were killed by 6-hydroxydopamine (6-OHDA), a neurotoxic catecholamine. Upon plating, these cells released dopamine and other catecholamines in response to K+ depolarization. Surviving TH+ neurons, derived from the cells differentiated in serum-free suspension cultures, were detected 8 weeks after transplantation into 6-OHDA-lesioned rat brains. This work suggests that hESCs can differentiate in simple serum-free suspension cultures to produce the large number of cells required for transplantation studies. | | 15579641
 |
beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease.
Masliah, E; Rockenstein, E; Veinbergs, I; Sagara, Y; Mallory, M; Hashimoto, M; Mucke, L
Proceedings of the National Academy of Sciences of the United States of America
98
12245-50
2001
Afficher le résumé
Alzheimer's disease and Parkinson's disease are associated with the cerebral accumulation of beta-amyloid and alpha-synuclein, respectively. Some patients have clinical and pathological features of both diseases, raising the possibility of overlapping pathogenetic pathways. We generated transgenic (tg) mice with neuronal expression of human beta-amyloid peptides, alpha-synuclein, or both. The functional and morphological alterations in doubly tg mice resembled the Lewy-body variant of Alzheimer's disease. These mice had severe deficits in learning and memory, developed motor deficits before alpha-synuclein singly tg mice, and showed prominent age-dependent degeneration of cholinergic neurons and presynaptic terminals. They also had more alpha-synuclein-immunoreactive neuronal inclusions than alpha-synuclein singly tg mice. Ultrastructurally, some of these inclusions were fibrillar in doubly tg mice, whereas all inclusions were amorphous in alpha-synuclein singly tg mice. beta-Amyloid peptides promoted aggregation of alpha-synuclein in a cell-free system and intraneuronal accumulation of alpha-synuclein in cell culture. beta-Amyloid peptides may contribute to the development of Lewy-body diseases by promoting the aggregation of alpha-synuclein and exacerbating alpha-synuclein-dependent neuronal pathologies. Therefore, treatments that block the production or accumulation of beta-amyloid peptides could benefit a broader spectrum of disorders than previously anticipated. | | 11572944
 |