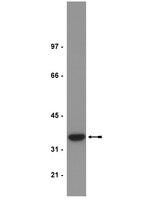
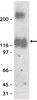
NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink.
Ulanovskaya, OA; Zuhl, AM; Cravatt, BF
Nature chemical biology
9
300-6
2013
Afficher le résumé
Nicotinamide N-methyltransferase (NNMT) is overexpressed in a variety of human cancers, where it contributes to tumorigenesis by a mechanism that is still poorly understood. Here we show using metabolomics that NNMT impairs the methylation potential of cancer cells by consuming methyl units from S-adenosyl methionine to create the stable metabolic product 1-methylnicotinamide. As a result, NNMT-expressing cancer cells have an altered epigenetic state that includes hypomethylated histones and other cancer-related proteins combined with heightened expression of protumorigenic gene products. Our findings thus point to a direct mechanistic link between the deregulation of a metabolic enzyme and widespread changes in the methylation landscape of cancer cells. | Western Blotting | 23455543
 |
Antiadrenergic effects of adenosine A(1) receptor-mediated protein phosphatase 2a activation in the heart.
Qinghang Liu, Polly A Hofmann, Qinghang Liu, Polly A Hofmann
American journal of physiology. Heart and circulatory physiology
283
H1314-21
2002
Afficher le résumé
The ability of adenosine A(1) receptors to activate type 2a protein phosphatase (PP2a) and account for antiadrenergic effects was investigated in rat myocardial preparations. We observed that the adenosine A(1) receptor agonist N(6)-cyclopentyladenosine (CPA) significantly reduces the isoproterenol-induced increase in left ventricular developed pressure of isolated heats, and this effect is blocked by pretreatment of hearts with the PP2a inhibitor cantharidin. CPA alone or given in conjunction with isoproterenol stimulation decreases phosphorylation of phospholamban and troponin I in ventricular myocytes. These dephosphorylations are blocked by an adenosine A(1) receptor antagonist and by PP2a inhibition with okadaic acid. Adenosine A(1) receptor activation was also shown to increase carboxymethylation of the PP2a catalytic subunit (PP2a-C) and cause translocation of PP2a-C to the particulate fraction in ventricular myocytes. These results support the hypothesis that adenosine A(1) receptor activation leads to methylation of PP2a-C and subsequent translocation of the PP2a holoenzyme. Increases in localized PP2a activity lead to dephosphorylation of key cardiac proteins responsible for the positive inotropic effects of beta-adrenergic stimulation. | | 12234781
 |
WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A.
Moreno, C S, et al.
J. Biol. Chem., 275: 5257-63 (2000)
1999
Afficher le résumé
Protein phosphatase 2A (PP2A) is a multifunctional serine/threonine phosphatase that is critical to many cellular processes including development, neuronal signaling, cell cycle regulation, and viral transformation. PP2A has been implicated in Ca(2+)-dependent signaling pathways, but how PP2A is targeted to these pathways is not understood. We have identified two calmodulin (CaM)-binding proteins that form stable complexes with the PP2A A/C heterodimer and may represent a novel family of PP2A B-type subunits. These two proteins, striatin and S/G(2) nuclear autoantigen (SG2NA), are highly related WD40 repeat proteins of previously unknown function and distinct subcellular localizations. Striatin has been reported to associate with the post-synaptic densities of neurons, whereas SG2NA has been reported to be a nuclear protein expressed primarily during the S and G(2) phases of the cell cycle. We show that SG2NA, like striatin, binds to CaM in a Ca(2+)-dependent manner. In addition to CaM and PP2A, several unidentified proteins stably associate with the striatin-PP2A and SG2NA-PP2A complexes. Thus, one mechanism of targeting and organizing PP2A with components of Ca(2+)-dependent signaling pathways may be through the molecular scaffolding proteins striatin and SG2NA. | | 10681496
 |
A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A.
Ogris, E, et al.
J. Biol. Chem., 274: 14382-91 (1999)
1998
Afficher le résumé
Carboxymethylation of proteins is a highly conserved means of regulation in eukaryotic cells. The protein phosphatase 2A (PP2A) catalytic (C) subunit is reversibly methylated at its carboxyl terminus by specific methyltransferase and methylesterase enzymes which have been purified, but not cloned. Carboxymethylation affects PP2A activity and varies during the cell cycle. Here, we report that substitution of glutamine for either of two putative active site histidines in the PP2A C subunit results in inactivation of PP2A and formation of stable complexes between PP2A and several cellular proteins. One of these cellular proteins, herein named protein phosphatase methylesterase-1 (PME-1), was purified and microsequenced, and its cDNA was cloned. PME-1 is conserved from yeast to human and contains a motif found in lipases having a catalytic triad-activated serine as their active site nucleophile. Bacterially expressed PME-1 demethylated PP2A C subunit in vitro, and okadaic acid, a known inhibitor of the PP2A methylesterase, inhibited this reaction. To our knowledge, PME-1 represents the first mammalian protein methylesterase to be cloned. Several lines of evidence indicate that, although there appears to be a role for C subunit carboxyl-terminal amino acids in PME-1 binding, amino acids other than those at the extreme carboxyl terminus of the C subunit also play an important role in PME-1 binding to a catalytically inactive mutant. | | 10318862
 |