Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery.
Santagata, S; Eberlin, LS; Norton, I; Calligaris, D; Feldman, DR; Ide, JL; Liu, X; Wiley, JS; Vestal, ML; Ramkissoon, SH; Orringer, DA; Gill, KK; Dunn, IF; Dias-Santagata, D; Ligon, KL; Jolesz, FA; Golby, AJ; Cooks, RG; Agar, NY
Proceedings of the National Academy of Sciences of the United States of America
111
11121-6
2014
Afficher le résumé
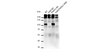
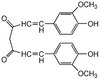
For many intraoperative decisions surgeons depend on frozen section pathology, a technique developed over 150 y ago. Technical innovations that permit rapid molecular characterization of tissue samples at the time of surgery are needed. Here, using desorption electrospray ionization (DESI) MS, we rapidly detect the tumor metabolite 2-hydroxyglutarate (2-HG) from tissue sections of surgically resected gliomas, under ambient conditions and without complex or time-consuming preparation. With DESI MS, we identify isocitrate dehydrogenase 1-mutant tumors with both high sensitivity and specificity within minutes, immediately providing critical diagnostic, prognostic, and predictive information. Imaging tissue sections with DESI MS shows that the 2-HG signal overlaps with areas of tumor and that 2-HG levels correlate with tumor content, thereby indicating tumor margins. Mapping the 2-HG signal onto 3D MRI reconstructions of tumors allows the integration of molecular and radiologic information for enhanced clinical decision making. We also validate the methodology and its deployment in the operating room: We have installed a mass spectrometer in our Advanced Multimodality Image Guided Operating (AMIGO) suite and demonstrate the molecular analysis of surgical tissue during brain surgery. This work indicates that metabolite-imaging MS could transform many aspects of surgical care. | 24982150
 |
Establishment of a novel monoclonal antibody SMab-1 specific for IDH1-R132S mutation.
Kaneko, Mika Kato, et al.
Biochem. Biophys. Res. Commun., 406: 608-13 (2011)
2010
Afficher le résumé
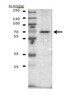
Isocitrate dehydrogenase 1 (IDH1) mutations, which are early and frequent genetic alterations in gliomas, are specific to a single codon in the conserved and functionally important Arginine 132 (R132) in IDH1. We earlier established a monoclonal antibody (mAb), IMab-1, which is specific for R132H-containing IDH1 (IDH1-R132H), the most frequent IDH1 mutation in gliomas. To establish IDH1-R132S-specific mAb, we immunized mice with R132S-containing IDH1 (IDH1-R132S) peptide. After cell fusion using Sendai virus envelope, IDH1-R132S-specific mAbs were screened in ELISA. One mAb, SMab-1, reacted with the IDH1-R132S peptide, but not with other IDH1 mutants. Western-blot analysis showed that SMab-1 reacted only with the IDH1-R132S protein, not with IDH1-WT protein or IDH1 mutants, indicating that SMab-1 is IDH1-R132S-specific. Furthermore, SMab-1 specifically stained the IDH1-R132S-expressing glioblastoma cells in immunocytochemistry and immunohistochemistry, but did not react with IDH1-WT or IDH1-R132H-containing glioblastoma cells. We newly established an anti-IDH1-R132S-specific mAb SMab-1 for use in diagnosis of mutation-bearing gliomas. | 21352804
 |
A monoclonal antibody IMab-1 specifically recognizes IDH1R132H, the most common glioma-derived mutation.
Kato, Yukinari, et al.
Biochem. Biophys. Res. Commun., 390: 547-51 (2009)
2009
Afficher le résumé
IDH1 (isocitrate dehydrogenase 1) mutations have been identified as early and frequent genetic alterations in astrocytomas, oligodendrogliomas, and oligoastrocytomas as well as secondary glioblastomas. In contrast, primary glioblastomas very rarely contain IDH1 mutations, although primary and secondary glioblastomas are histologically indistinguishable. The IDH1 mutations are remarkably specific to a single codon in the conserved and functionally important Arg132 in IDH1. In gliomas, the most frequent IDH1 mutations (>90%) were G395A (R132H). In this study, we immunized mice with R132H-containing IDH1 (IDH1(R132H)) peptide. After cell fusion using Sendai virus envelope, the monoclonal antibodies (mAbs), which specifically reacted with IDH1(R132H), were screened in ELISA. One of the mAbs, IMab-1 reacted with the IDH1(R132H) peptide, but not with wild type IDH1 (IDH1(wt)) peptide in ELISA. In Western-blot analysis, IMab-1 reacted with only the IDH1(R132H) protein, not IDH1(wt) protein or the other IDH1 mutants, indicating that IMab-1 is IDH1(R132H)-specific. Furthermore, IMab-1 specifically stained the IDH1(R132H)-expressing cells in astrocytomas in immunohistochemistry, whereas it did not react with IDH1(R132H)-negative primary glioblastoma sections. In conclusion, we established an anti-IDH1(R132H)-specific monoclonal antibody IMab-1, which should be significantly useful for diagnosis and biological evaluation of mutation-bearing gliomas. | 19818334
 |